Abstract
Mixtures of polyglycerol polyglycidyl ether (PGPE) and poly(ethylene glycol) diglycidyl ether (PEGDGE) with different molar ratios were cured with imine-containing phenolic hardeners prepared by the reactions of vanillin with ethylene glycol bis(3-aminopropyl) ether, diethylene glycol bis(3-aminopropyl) ether, and a polyetheramine (JEFFAMINE® ED-600) to produce bio-based epoxy cured products. Fourier-transform infrared spectroscopy (FT-IR) of the cured products revealed that the curing reaction of the epoxy and phenolic hydroxy groups was almost complete. The cross-linking density, glass transition temperature, and mechanical strength of the cured products decreased with decreasing the PGPE/PEGDGE ratio and increasing the oligoalkyleneoxy chain length of the phenolic hardeners. All cured products were healed three times at 100 °C under 2 MPa for 2 h. The healing efficiency, in terms of tensile strength, increased with decreasing PGPE/PEGDGE ratio and increasing oligoalkyleneoxy chain length. The polyetheramine-based cured product with the lowest PGPE/PEGDGE ratio exhibited the highest healing efficiency (94–97%), which only slightly decreased following repeated healing treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bio-based vitrimers are renewable biomass resource-derived polymer networks containing exchangeable dynamic covalent bonds, such as esters, imines, boronic acid esters, and disulfide bonds. They have attracted considerable attention because of their healing ability and reprocessability, therefore contributing to the saving of fossil fuel resources and carbon neutrality at a level that conventional thermosets cannot achieve [1,2,3,4,5,6]. Vanillin (VN) is a promising compound for the preparation of bio-based vitrimers because it is currently one of the only industrially available bio-based aromatic compounds [7], and its aldehyde group can be easily converted to an exchangeable imine bond through a reaction with primary amine compounds [8, 9]. Several studies have been conducted on VN-based vitrimers containing exchangeable imine bonds. For example, Wang et al. reported imine-containing epoxy vitrimers prepared by curing a VN-epichlorohydrin reaction product, 3-methoxy-4-(oxiran-2-ylmethoxy) benzaldehyde (MB), with 4,4’-methylenebiscyclohexanediamine, which exhibited reprocessable properties by treating at 180 °C for 20 min under 15 MPa [10]. Yu et al. reported imine-containing epoxy vitrimers prepared by curing MB with isophorone diamine, which exhibited reprocessable properties by treating at 130 °C for 5 min under 10 MPa [11]. Liu et al. reported bio-based epoxy vitrimers prepared by curing epoxidized soybean oil (ESO) with an imine-containing bisphenol derived from VN and p-aminophenol, which exhibited reprocessable properties by treating at 180 °C under 20 MPa [12]. Veloso-Fernandez et al. reported imine-containing epoxy vitrimers, prepared by curing ESO with an imine-containing bisphenol derived from VN and 1,4-diaminobutane, which exhibited reprocessability by treating at 150 °C and 10 bar for 1 h [13]. Chen et al. reported bio-based epoxy vitrimers prepared by VN-derived imine-containing di- and tri-functional epoxy resins and a polyether amine (JEFFAMINE® D400), which exhibited healable and welding abilities by treating at 85 °C for 15 min [14]. Memon et al. reported bio-based epoxy vitrimers, prepared by curing mixtures of N,N-diglycidyl-4-glycidyoxyaniline and butanediol diglycidyl ether with an imine-containing hardener derived from VN and isophorone diamine, which exhibited reprocessability by treating at 200 °C for 30 min under 0.3 MPa [15]. Roig et al. reported imine-containing epoxy vitrimers prepared by the reactions of a mixture of VN-derived diamine-diglycidyl derivative/trimethylolpropane triglycidyl ether (molar ratio:3/1) and three different polyetheramines (JEFFAMINE® T-403, D230, and D400), which displayed reprocessing and self-welding abilities by treating at 180 °C for 2 h under 3 MPa [16]. Rasid et al. reported imine-containing epoxy vitrimers prepared by curing bisphenol-F epoxy resin and neopentyl glycol diglycidyl ether with an imine-containing epoxy hardener derived from VN and 1,3-bis(aminomethyl)cyclohexane. The epoxy vitrimer displayed reprocessing abilities by treating at 170 °C for 30 min under 0.3 MPa [17]. The aforementioned vitrimers either did not exhibit healing, or such property was not reported. Liu et al. reported an imine-containing epoxy vitrimer prepared by curing diglycidyl ether of bisphenol A (DGEBA) with a bisphenol derived from VN and m-phenylene diamine in the presence of 2-ethyl-4-methylimidazole. Although pressing the vitrimer at 10 N and 120 °C resulted in healing properties with a healing efficiency of 61% in terms of tensile strength, their repetitive healing properties and the influence of compositional change on such properties were not reported [18]. Therefore, it is important to elucidate the influence of bio-based epoxy resins and hardeners with different chemical structures and properties, (i.e. imine content, cross-linking density, and flexibility of the cured products) on the thermal, mechanical, and repetitive healing properties of imine-containing vitrimers.
In this study, mixtures of polyglycerol polyglycidyl ether (PGPE), a bio-based glycerol-derived epoxy resin with a functionality of approximately 4, and poly(ethylene glycol) diglycidyl ether (PEGDGE), a flexible epoxy resin with a functionality of 2, prepared using different molar ratios, were cured with imine-containing phenolic hardeners (BVEG, BVDEG, and BVJA). The hardeners were prepared by the reactions of VN with ethylene glycol bis(3-aminopropyl) ether (EGBE), diethylene glycol bis(3-aminopropyl) ether (DEGBE), and a polyetheramine (JA), respectively (Scheme 1 and 2). The influence of the different chain lengths and epoxy functionalities of these imine-containing phenolic hardeners on the thermal, mechanical, and healing properties of the cured products were systematically studied.
Experimental
Materials
PGPE (trade name: DENACOL® EX-512, with an epoxy equivalent weight of 167 g/eq., average functional group number of 4, chlorine content of 6.5%, viscosity of 1,300 mPa s at 25 °C) was supplied by Nagase ChemteX. Corp. (Tokyo, Japan). PEGDGE (average Mn:500) was purchased from Sigma–Aldrich Japan Co., Ltd. (Tokyo, Japan). VN, EGBE, and DEGBE were purchased from Tokyo Chemical Industry (Tokyo, Japan). JA (trade name:JEFFAMINE® ED-600, average molecular weight ca. 600, amine (NH2) equivalent 312.5 g/eq.) was supplied from TOMOE Engineering CO., Ltd. (Tokyo, Japan). Triphenyl phosphine (TPP) was purchased from FUJIFILM Wako Pure Chemical Co., Ltd. (Tokyo, Japan). Commercially available reagents were used as received, without further purification.
Preparation of phenolic hardeners containing imine bonds
Typic synthetic procedure of BVEG is as follows: VN (12.2 g, 80.0 mmol) was added to a solution of EGBE (7.05 g, 40.0 mmol) in chloroform, and the resulting mixture was stirred under a nitrogen atmosphere at 35 °C for 6 h. The reaction mixture was dried over sodium sulfate, and then filtered. The crude product was precipitated with hexane, and the supernatant was decanted off. The isolated precipitate was washed with diethyl ether three times by decantation, dried at 120 °C in a vacuum oven for 24 h to give BVEG as a reddish brown solid (yield: 16.2 g, 91%). BVDEG and BVJA were synthesized by the same procedure except that dichloromethane was used instead of chloroform as reddish brown solid and liquid in yields of 90% and 80%, respectively. BVEG: 1H-NMR (δ, ppm, in dimethyl sulfoxide-d6 (DMSO-d6)) 8.32 (s, 2H, H-e), 7.32 (d, 2H, H-c, Jcb = 2 Hz), 7.09 (dd, 2H, H-b, Jba = 8 Hz, Jbc = 2 Hz), 6.81 (d, 2H,H-a, Jab = 8 Hz), 3.78 (s, 6H,H–d), 3.78–3.17 (m, 12H, H-f, h,i), and 1.82 (m, 4H, H-g); FT-IR (cm–1) 3350–3100, 2920, 2857, 1639, 1585, 1510, 1462, 1425, 1333, 1277, 1234, 1200, 1134, 1117, 1024, 964, 860, 818, 743, 610, and 571. BVDEG: 1H-NMR (δ, ppm, in DMSO-d6) 8.16 (s, 2H, H-e), 7.32 (sl, 2H, H-c), 7.09 (dl, 2H, H-b, Jba = 8 Hz), 6.81 (d, 2H,H-a, Jab = 8 Hz), 3.79 (s, 6H,H–d), 3.55–3.42 (m, 16H, H-f, h, i, j), and 1.82 (m, 4H, H-g); FT-IR (cm–1) 3300–3100, 2916, 2859, 1639, 1589, 1512, 1462, 1423, 1334, 1279, 1101, 1026, 860, 820, 743, 611, and 575. BVJA: 1H-NMR (δ, ppm, in DMSO-d6) 8.17 (s, 2H, H-e), 7.32 (sl, 2H, H-c), 7.09 (dl, 2H, H-b, Jba = 8 Hz), 6.81 (d, 2H,H-a, Jab = 8 Hz), 3.79 (s, 6H,H–d), 3.6–3.2 (m, 52.3H, H-f, g, i, j, l), 1.12 (d, 5.9H, H–k, Jki = 6 Hz), and 1.04, 0.98 (d, d, 5.3H, H–h, Jhf = 6 Hz); FT-IR (cm–1) 3350–3100, 2862, 1639, 1589, 1512, 1506, 1429, 1348, 1282, 1092, 1030, 943, 864, 820, 744, 621, and 575.
Preparation of cured epoxy products
A typical preparation method of a PGPE/PEGDGE/BVEG cured product at the epoxy/OH ratio of 1/1 and PGPE/PEGDGE epoxy ratio of 1/1 (BVEG-PG/PEG-1/1) is as follows: A mixture of BVEG (3.09 g, 13.9 mmol-OH), PGPE (1.17 g, 6.95 mmol-epoxy), PEGDGE (1.74 g, 6.95 mmol-epoxy), and TPP (1 wt% of total wight) was stirred at 140 °C until the mixture becomes to a gelatinous material (ca. 5–10 min). The pre-polymer solid was compression-molded at 180 °C under 3 MPa for 2 h using Mini Test Press-10 (Toyo Seiki Co., Ltd, Tokyo, Japan) to produce BVEG-PG/PEG-1/1. Other cured products were prepared by a similar method to BVEG-PG/PEG-1/1 except that the prepolymerization and compression molding conditions for BVJA-PG/PEG were 180 °C (ca. 30–40 min) and 190 °C for 3 h, respectively. Sample abbreviations, amounts of feed reactants, and imine contents of all cured products are listed in Table 1. All cured epoxy products were obtained as homogenous brown films.
Healing of cured products
Rectangular samples (45 × 7 × 0.4–0.7 mm3) of the cured products were cut into halves. The two pieces were contacted at the cross-section at around 100 °C for 10 s, sandwiched between two Teflon sheets and stainless steel plates, and hot-pressed at 100 °C under 2 MPa for 2 h using a Mini Test Press-10 to obtain healed BVEG-PG/PEG, BVDEG-PG/PEG, and BVJA-PG/PEG specimens (h1-BVEG-PG/PEG, h1-BVDEG-PG/PEG, and h1-BVJA-PG/PEG). This healing cycle was repeated three times (h1, h2, and h3). The healing efficiency in tensile of tensile strength (ησ) was calculated from the tensile strength recovery rate using the following equation:
where σ0 and σ1 are the average tensile strengths of the original and healed samples, respectively.
Measurements
Fourier-transform infrared (FT-IR) spectra were recorded on a Shimadzu (Kyoto, Japan) IRAffinity-1S in the 4000–500 cm−1 range using the attenuated total reflectance (ATR) method. The IR spectra were acquired using 50 scans at a resolution of 4 cm−1.
Proton nuclear magnetic resonance (1H-NMR) spectra were recorded with a Bruker Ascend 400 MHz spectrometer (Madison, WI, USA) using DMSO-d6 as the solvent.
To quantify the degree of swelling (Ds), the film (10 × 10 × 0.4–0.7 mm3) was dipped in chloroform at room temperature for 24 h. The following equation was used:
where w0 is the initial weight of the film and w1 is the weight of the swollen film after dipping. The film dipped in chloroform was dried at 100 °C in a vacuum oven for 24 h, and the gel fraction (Gf) by chloroform extraction was calculated using the following equation:
where w0 and w2 are the weights of the original and dried films, respectively. Three samples of each film were tested, and the mean values and standard deviations were calculated from the degree of swelling and gel fraction measurements.
Differential scanning calorimetry (DSC) was performed using a Shimadzu DSC-60Plus instrument under a nitrogen atmosphere. After the as-prepared sample (5–8 mg) was cooled to − 80 °C, the heating scan was monitored at a heating rate of 20 °C min−1. The glass transition temperature (Tg) was determined from the mid-point of the change in heat flow.
Dynamic mechanical analysis (DMA) (DMA1, Mettler–Toledo, Japan) on a rectangular plate sample (20 × 5 × 0.4–0.7 mm) was performed with a chuck distance of 10 mm, a frequency of 1 Hz, and a heating rate of 10 °C min−1. The amplitude for the DMA measurements were 7 μm. The loss tangent (tan δ) peak temperature (Tα) ascribed to glass transition was obtained from the temperature dependency of tan δ. The crosslinking density (ve) of the cured products was calculated from the following equation:
where R is the gas constant, T is the absolute temperature, and E′ is the storage modulus in the rubbery state. To ensure that the cured resins were in the rubbery state, the temperature at which E′ was taken was set at Tα + 30 °C for each sample [15, 19].
Thermogravimetric analysis (TGA) was performed for a sample weighing approximately 5 mg using a Shimadzu TGA-50 thermogravimetric analyzer at a heating rate of 20 °C min–1 in a nitrogen atmosphere. The temperatures at which x% mass loss occurred (Tdx%, x = 5, 10, and 50) were determined using the TGA curves.
Tensile tests of rectangular samples (45 × 7 × 0.4–0.7 mm3) were performed at a temperature of 20–25 °C, using a Shimadzu Autograph AGS-X instrument. The span length and testing speed were 25 mm and 5 mm min−1, respectively. Five specimens were tested for each set of samples and the mean values and standard deviations were calculated.
Results and discussion
Synthesis and characterization of imine-containing phenolic hardeners
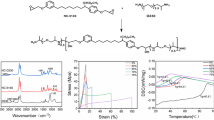
BVEG, BVDEG, and BVJA were prepared by the condensation reactions of VN with EGBE, DEGBE, and JA, respectively (Scheme 1). Figure 1 shows the FT-IR spectra of BVEG, BVDEG, and BVJA along with those of EGBE, DEGBE, and JA. The spectrum of VN exhibits the presence of absorption bands corresponding to phenolic hydroxy (OH) and aldehyde carbonyl (C = O) stretching vibrations (νOH and νC=O) at 3147 and 1660 cm‒1, respectively, whereas the spectra of EGBE, DEGBE, and JA reveal the presence of absorption bands attributed to the primary amine (NH2) stretching (νNH) and scissoring vibrations (δNH) at 3364–3368 and 1599 cm‒1, respectively. The bands characteristic of C = O and NH2 groups were not detected in the FT-IR spectra of BVEG, BVDEG, and BVJA, and the absorption bands corresponding to νOH and the imine C = N stretching vibration (νC=N) were observed at approximately 3350–3100 and 1639 cm–1, respectively. These results indicate that BVEG, BVDEG, and BVJA contain phenolic hydroxy groups and imine bonds that are formed following the condensation reaction of the aldehyde group of VN with the primary amino groups of EGBE, DEGBE, and JA.
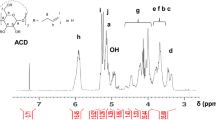
Figure 2 shows the 1H-NMR spectra of BVEG, BVDEG, and BVJA in DMSO-d6. BVEG, BVDEG, and BVJA displayed the 1H-NMR chemical shift (δ) of imine protons at 8.32–8.16 ppm (H-e), those of aromatic protons at 7.32 (s, 2H, H-c), 7.09 (dd or dl, 2H, H-b), and 6.81 ppm (d, 2H, H-a), and those of methoxy protons at 3.79–3.78 ppm (s, 6H, H–d), in addition to those of alkyleneoxy protons at 3.8–1.0 ppm. The chemical structure of BVEG, BVDEG, and BVJA was thus confirmed from the FT-IR and 1H-NMR analyses.
Preparation and characterization of epoxy cured products
Mixtures of PGPE/PEGDGE at the epoxy molar ratios of 1/0, 3/1, and 1/1 were prepolymerized with either BVEG, BVDEG, or BVJA at 140–180 °C, at the epoxy/phenolic OH ratio of 1/1, and then compression-molded at 180–190 °C to generate the cured products (BVEG-PG/PEG-1/0, 3/1, and 1/1, BVDEG-PG/PEG-1/0, 3/1, and 1/1, or BVJA-PG/PEG-1/0, 3/1, and 1/1) as pale brown films (Scheme 2). Figure 3 shows the FT-IR spectra of the BVEG/PEG-cured products and their reactants (DVEG, PGPE, and PEGDGE) whereas those of BVDEG-PG/PEG and BVJA-PG/PEG films are shown in Fig. S1 and S2, respectively (see Supplementary Material). The spectra of PGPE and PEGDGE display absorption bands characteristic of epoxy groups at 907, 839, and 756 cm‒1 whereas they were not present in those of BVEG-PG/PEG films. However, new alcoholic νOH bands are observed at approximately 3400 cm‒1, indicating that the epoxy-phenolic hydroxy curing reaction proceeded as shown in Scheme 2. Similarly, the epoxy-phenolic hydroxy curing reaction was confirmed for the BVDEG-PG/PEG and BVJA-PG/PEG films (Fig. S1 and S2).
Figure 4 shows the degree of swelling (Ds) and gel fraction (Gf) values of all the cured products obtained using chloroform as the dipping solvent. Ds increases with decreasing PGPG/PEGDGE ratio, suggesting that the cross-linking density decreases by the same order of magnitude. In addition, a decrease of Gf is observed with decreasing PGPG/PEGDGE ratio, indicating that the reactions of PEGDGE with BVEG, BVDEG, or BVJA generated linear polymers that are soluble in chloroform. When cured films with the same PGPG/PEGDGE ratio are compared, Ds increases in the following order: BVEG-PG/PEG < BVDEG-PG/PEG < BVJA-PG/PEG, indicating that the cross-linking density should decrease as the alkyleneoxy chain lengths of the phenolic hardeners increase (i.e. BVEG < BVDEG < BVJA). Moreover, Gf decreases in the following order: BVEG-PG/PEG > BVDEG-PG/PEG > BVJA-PG/PEG, indicating that the feed weight fraction of the difunctional phenolic hardeners should increase by the same order of magnitude.
Thermal properties of the cured products
Table 2 summarizes the glass transition temperature (Tg) values obtained from the DSC heating curves (see Fig. S3 (Supplementary Material)). It is observed that Tg values are lower for lower PGPG/PEGDGE ratio, indicating a reduction of the cross-linking density. Tg values are likewise seen to decrease in the following order: BVEG-PG/PEG > BVDEG-PG/PEG > BVJA-PG/PEG, also indicating a reduction of the cross-linking density. The Tg values (–12.7 to –3.6 °C) of BVJA-PG/PEG films are much lower than those of BVEG-PG/PEG (29.2 to 65.9 °C) and BVDEG-PG/PEG (17.0 to 51.7 °C) films, indicating that JA has much longer and flexible alkyleneoxy chains than EGBE and DEGBE.
Figure 5 shows the typical temperature dependence of the E′ and tan δ obtained by DMA measurements of BVEG-PG/PEG cured products. The DMA curves of BVDEG-PG/PEG and BVJA-PG/PEG cured products were shown in Fig. S4 (see Supplementary Material). The Tα values obtained from the DMA curves are summarized in Table 2. The E′ rubbery plateau region is observed for all the cured products at temperatures higher than Tα, indicating that the network structure is formed. Both Tα and the νe values decreased in the following order: BVEG-PG/PEG > BVDEG-PG/PEG > BVJA-PG/PEG with decreasing PGPG/PEGDGE ratio, in agreement with the trends observed for Tg and Ds.
Table 2 summarizes the Tdx% (x = 5, 10, and 50) values for all cured products. The TGA curves of all the cured products and reactants (VN, EGBE, DEGBE, and JA) are shown in Fig. S5 and S6, respectively (see Supplementary Material). There is no significant differences in Tdx% (x = 5, 10, and 50) values associated with changes in the PGPE/PEGDGE ratio, the Tdx% (x = 5, 10, and 50) values of the BVEG-PG/PEG films being comparable to those of the BVDEG-PG/PEG films. However, the Tdx% (x = 5 and 10) values of the BVJA-PG/PEG films are higher than those of the BVEG-PG/PEG and BVDEG-PG/PEG films, which most probably results from the higher Td5% and Td10% (273 and 298 °C) values of JA as compared to those of EGBE (70 and 97 °C) and DEGBE (63 and 85 °C). The TGA curves for EGBE, DEGBE, and JA are shown in Fig. S4 (see Supplementary Materials).
Mechanical and healing properties of cured products
Figure 6 shows the tensile stress–strain curves of all cured products. The tensile strength, tensile modulus, and elongation at break obtained from these curves are summarized in Table 3. The tensile strength and modulus both decrease with decreasing PGPG/PEGDGE ratio in following order: BVEG-PG/PEG > BVDEG-PG/PEG > BVJA-PG/PEG, in agreement with the trends observed for Tg and Tα. In contrast, the elongation at break increased by a similar order of magnitude, but it is observed to be higher for BVDEG-PG/PEG-3/1 than for BVDEG-PG/PEG-1/1. The tensile strength and modulus (0.38–0.89 and 1.33–3.83 MPa) of BVJA-PG/PEG films are much lower than those of BVEG-PG/PEG (15.7–80.6 and 453–2533 MPa) and BVDEG-PG/PEG (5.4–72.6 and 52.5–2220 MPa) films, indicating that JA has a much longer flexible alkyleneoxy chain as compared to EGBE and DEGBE.
The healing behavior of cross-shaped scratches formed on the surfaces of the cured films were investigated by pressing under 2 MPa at 100 °C for 2 h (Fig. 7). The original scratches on the cured films narrowed with increasing softness, especially those on the BVJA-PG/PEG film, which are significantly narrower than those on the BVEG-PG/PEG and BVJA-PG/PEG films. The cross-shaped scratches became smaller following the healing treatment, and the healing ability increases with decreasing PGPE/PEGDGE ratio. No differences are observed in the healing ability of scratches among BVEG-PG/PEG, BVJA-PG/PEG, and BVJA-PG/PEG films with the same PGPE/PEGDGE ratio.
The healing properties of the cured products are also quantitatively evaluated based on the changes in the tensile properties of the original and healed samples. The as-prepared samples of the cured films are first cut into two pieces and then contacted from the cross-section at 100 °C for 10 s and then pressed at 100 °C under 2 MPa for 2 h, thereby adhering together to form a healed (h1) film. The h1-samples are healed twice using the same procedure to produce h2- and h3-samples. Typical tensile stress–strain curves of the h1-, h2-, and h3-BVDEG-PG/PEG samples are shown in Fig. 8, whereas those of h1-, h2-, and h3-BVEG-PG/PEG and h1-, h2-, and h3-BVJA-PG/PEG samples are shown in Fig. S7 and Fig. S8, respectively (see Supplementary Materials). The maximum tensile stress and strain at break are seen to gradually decrease as the number of healing cycles increases.
The healing efficiency (ησ), in terms of tensile strength, for all healed samples are shown in Fig. 9 whereas Table S1 summarizes the tensile strength, tensile modulus, and elongation at break of h1, h2, and h3-samples (see Supplementary Materials). The ησ of healed samples decreases with increasing healing number (i.e., h1 > h2 > h3) whereas it increases with decreasing PGPE/PEGDGE ratio (i.e., PG/PEG-1/0 < -3/1 < -1/1) and increasing chain length of the phenolic hardener component (i.e., BVEG-PG/PEG < BVDEG-PG/PEG < BVJA-PG/PEG). The ησ of h1-BVJA-PG/PEG samples are higher than 92% and the decrease of ησ with increasing healing number is very small for h1-, h2-, and h3-BVJA-PG/PEG-1/1 samples (97.1%, 96.7%, and 94.8%, respectively), which have the lowest imine content. The healing efficiency of this curing process is thus seen to improve with an increase in flexibility and a reduction of the cross-linking density, thereby facilitating the imine metathesis reaction, irrespective of the small difference in imine content.
Conclusions
Mixtures of PGPE/PEGDGE with epoxy molar ratios of 1/0, 3/1, and 1/1 were cured with three types of phenolic hardeners (BVEG, BVDEG, or BVJA) by compression molding to produce BVEG-PG/PEG-, BVDEG-PG/PEG-, or BVJA-PG/PEG-cured products. The effects of the chain length of the phenolic hardeners and epoxy functionalities on the thermal, mechanical, and healing properties of the bio-based epoxy networks were investigated. FT-IR and gel fraction analyses of the cured products revealed that the curing reaction of the epoxy and phenolic hydroxy groups proceeded almost completely to produce network polymers. The DSC, DMA, and tensile tests revealed that the Tg, Tα, νe, and mechanical strength of the cured products decreased with decreasing PGPE/PEGDGE ratio and increasing oligoalkyleneoxy chain length of the phenolic hardeners. Following three healing treatments at 100 °C under 5 MPa for 2 h, the ησ of all cured products increased with decreasing PGPE/PEGDGE ratio and increasing oligoalkyleneoxy chain length. h1-, h2- and h3-BVJA-PG/PEG-1/1 samples exhibited the highest ησ (94–97%) with very limited reduction following repeated healing treatments. These epoxy networks thus have great potential for applications that require sustainable coating materials and small components with excellent healing properties.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Lucherelli MA, Duval A, Avérous L (2022) Biobased vitrimers: towards sustainable and adaptable performing polymer materials. Prog Polym Sci 127:101515. https://doi.org/10.1016/j.progpolymsci.2022.101515
Krishnakumar B, Pucci A, Wadgaonkar PP, Kumar I, Binder WH, Rana S (2022) Vitrimers based on bio-derived chemicals: overview and future prospects. Chem Eng J 433:133261. https://doi.org/10.1016/j.cej.2021.133261
Chong KL, Lai JC, Rahman RA, Adrus N, Al-Saffar ZH, Hassan A, Lim TH, Wahit MU (2022) A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils. Ind Crops Prod 189:115857. https://doi.org/10.1016/j.indcrop.2022.115857
Zhang Y, Ma F, Shi L, Lyu B, Ma J (2023) Recyclable, repairable, and malleable bio-based epoxy vitrimers: overview and future prospects. Curr Opin Green Sustain Chem 39:100726. https://doi.org/10.1016/j.cogsc.2022.100726
Yang Y, Lin H, Lin B, Tang D, Xu J, Dai L, Si C (2023) From biomass to vitrimers: Latest developments in the research of lignocellulose, vegetable oil, and naturally-occurring carboxylic acids. Ind Crops Prod 206:117690. https://doi.org/10.1016/j.indcrop.2023.117690
Sangermano M, Bergoglio M, Schögl S (2023) Biobased vitrimetric epoxy networks. Macromol Mater Engn 24:2300371. https://doi.org/10.1002/mame.202300371
Fache M, Boutevin B, Caillol S (2015) Vanillin, a key-intermediate of biobased polymers. Eur Polym J 68:488–502. https://doi.org/10.1016/j.eurpolymj.2015.03.050
Qiang H, Wang J, Liu H, Zhu Y (2023) From vanillin to biobased aromatic polymers. Polym Chem 14:4255. https://doi.org/10.1039/d3py00767g
Liguori A, Hakkarainen M (2022) Designed from biobased materials for recycling: imine-based covalent adaptable networks. Macromol Rapid Commun 43:2100816. https://doi.org/10.1002/marc.202100816
Wang S, Ma S, Li Q, Xu X, Wang B, Yuan W, Zhou S, You S, Zhu J (2019) Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem 21:1484. https://doi.org/10.1039/c8gc03477j
Yu Q, Peng X, Wang Y, Geng H, Xu A, Zhang X, Xu W, Ye D (2019) Vanillin-based degradable epoxy vitrimers: reprocessability and mechanical properties study. Eur Polym J 117:55–63. https://doi.org/10.1016/j.eurpolymj.2019.04.053
Liu YY, Li YD, Zhao XL, Zeng JB (2020) Biobased epoxy vitrimer from epoxidized soybean oil for reprocessable and recyclable carbon fiber reinforced composite. Compos Commun 22:100445. https://doi.org/10.1016/j.coco.2020.100445
Veloso-Fernández A, Ruiz-Rubio L, Yugueros I, Moreno-Benítez MI, Laza JM, Vilas-Vilela JL (2023) Improving the recyclability of an epoxy resin through the addition of new biobased vitrimer. Polymers 15:3737. https://doi.org/10.3390/polym15183737
Chen P, Ding Y, Wang Y, Zhao H, Li P, Liu Y, Gao C (2023) Functional bio-based vitrimer with excellent healing and recyclability based on conjugated deflection self-toughening. Chem Eng J 474:145680. https://doi.org/10.1016/j.cej.2023.145680
Memon H, Wei Y, Zhu C (2021) Correlating the thermomechanical properties of a novel bio-based epoxy vitrimer with its crosslink density. Mater Today Commun 29:102814. https://doi.org/10.1016/j.mtcomm.2021.102814
Roig A, Hidalgo P, Ramis X, Flor SD, Serra À (2022) Vitrimeric epoxy-amine polyimine networks based on a renewable vanillin derivative. ACS Appl Polym Mater 4:9341–9350. https://doi.org/10.1021/acsapm.2c01604
Rashid MA, Zhu S, Zhang L, Jin K, Liu W (2023) High-performance and fully recyclable epoxy resins cured by imine-containing hardeners derived from vanillin and syringaldehyde. Eur Polym J 187:111878. https://doi.org/10.1016/j.eurpolymj.2023.111878
Liu X, Liang L, Lu M, Song X, Liu H, Chen G (2020) Water-resistant bio-based vitrimers based on dynamic imine bonds: self-healability, remodelability and ecofriendly recyclability. Polymer 210:123030. https://doi.org/10.1016/j.polymer.2020.123030
Xu N, Kim S, Liu Y, Adraro YA, Li Z, Hu J, Liu L, Hu Z, Huang Y (2020) Facile preparation of rapidly recyclable tough thermosetting composites via cross-linking structure regulation. Polymer 189:122163. https://doi.org/10.1016/j.polymer.2020.122163
Acknowledgements
The authors acknowledge the financial support from the Chiba Institute of Technology.
Funding
Open access funding provided by Chiba Institute of Technology. The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Ryuki Kubota: Software, Formal analysis, Investigation, Writing-Original draft preparation, Visualization. Kaito Sugane: Software, Formal analysis, Investigation. Mitsuhiro Shibata: Conceptualization, Methodology, Validation, Resources, Data curation, Writing-Reviewing and Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubota, R., Sugane, K. & Shibata, M. Effect of imine-containing phenolic hardeners with different chain lengths and epoxy functionalities on thermal, mechanical, and healing properties of bio-based epoxy vitrimers. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05327-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05327-5