Abstract
The main object of this report is to review and summarize the current state of the scientific literature relating to physico-chemical ageing of ethylene–norbornene copolymers (ENCs). These processes occur through different means such as photodegradation under the effect of ultraviolet radiation, temperature and ionizing radiation (under air or oxygen). In most cases ageing causes chains scissions that lead to the creation of degradation compounds that exhibit high mobility, corresponding to their low molecular weight and/or high polarity. Cross-linking can also occur, leading to the modification of mechanical properties. The presence of oxygen during ageing is of great importance for the physico-chemical modifications of those polymers in terms of oxidation and thermal stability. ENCs belong to cyclic olefin copolymers (COC) class, whose synthesis was developed in the late 1950s. Nevertheless, commercial applications are relatively new. They attract growing interest in different fields, due to their very useful physical and chemical properties such as transparency, heat and chemical resistance, low permeability to gas as well as biocompatibility. In the literature, a large number of papers deal with the synthesis and characterization of different grades of ENC, but only a few deal with the effects of ageing on these materials. This literature review consists of a summary of the main milestones in the development of COC as well as of cyclic olefin polymers (COP), a review of main techniques for predicting ageing, degradation of additives and interaction between COC/COP and contact environment as well as toxicity of the extractables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copolymers based on cyclic olefins were firstly synthesized in the late 1950s but are relatively new commercially speaking. They exhibit very useful physico-chemical properties such as glass-like transparency, rigidity, heat and chemical resistance and low permeability to gas and water [1,2,3,4,5,6,7]. These features make cyclic olefin copolymers (COC) of interest for many applications. Due to their good optical and mechanical properties, COC are used in optics to make compact discs, magneto-optic storage discs, light grids and other optical devices. Thanks to their good dielectric resistance over a wide temperature range, COC can replace polypropylene in thin film capacitors. COC are also used in housing, gears and colour toner binder resin, to name a few applications.
We will focus on the medical and pharmaceutical applications of COC such as primary packaging for drugs in solid form (blisters) and in liquid form, particularly for injectable formulations [4, 8,9,10]. In addition, they are widely used in the field of diagnostics for microwell plates as well as microfluidic devices and biodiagnostic chips [11,12,13,14,15,16,17,18,19,20,21,22]. COC have a low density and low autofluorescence [12, 23]. As a result, they have attracted considerable recent attention as substrate for biochips [24, 25]. COC are also used for biosensor technology [26, 27], DNA immobilization [28], microarrays, nucleic acid purification and immunoassays [17, 29,30,31,32,33]. The use of a blend of COC with polyethylene for the creation of bone replacement materials has also been reported [34].
Studies have also been made in other fields, concerning the formation of COC nanoparticles [35], production of COC/silica nanocomposites foams [36] and the processability of COC yarn by melt spinning [37]. Very recently, a paper has been published by Saadé et al. [38], concerning the modification of cyclic olefin copolymer in order to conduct to new electrochromatographic stationary phases.
Depending on their use, several physico-chemical treatments undergone by these plastic materials can induce their ageing: among others sterilization, thermal treatment, photons interactions.
In the scientific literature, a large number of papers are dealing with the synthesis and characterization of the different grades of COC, but only a few papers deal with the effects of ageing on these materials.
The goal of this review is therefore to highlight this second part and to focus on a particular type of COC known as ethylene–norbornene copolymers (ENCs). We shall present the synthesis pathways of ENCs, the physico-chemical properties of their different commercial grades as well as the various additives added to ENCs. Finally, we will consider the degradation of those additives and we will end with the cytotoxicity of ENCs and their degradation products that can affect the material’s biocompatibility.
Cyclic olefin copolymers (COC)
Development of cyclic olefin copolymers (COC)
Cyclic olefin copolymers (COC) belong to the amorphous polymer class. These materials have become economically important in the recent years, although their first synthesis was done 40 years ago. Bibliographical references concerning the industrial synthesis of cyclic olefin copolymers firstly appeared in the late 1950s. The first commercial products, via the Ziegler–Natta catalysts [39, 40], became available at the end of the 1980s. In 1984, a particular type of cyclic olefin polymer (COP) that contains norbornene was carried out by Yamazaki and collaborators from Zeon Company in Japan [41]. COC and COP are very similar, with the exception that COP uses a single type of monomer during formulation.
The vinylic polymerization of norbornene with Pd(II) catalysts was patented in 1967, and much research has been done with different metal catalysts (Ni(II), Pd(II), Cr(III), Co(II), Cu(II)) [42,43,44,45,46]. In 1989, Kaminsky developed a process of synthesis using metallocene catalysts, in particular zirconocene and half-sandwich titanium methylaluminoxane (MAO) catalysts [47,48,49,50], allowing the development of a perfectly transparent material with a great rigidity, which was in the centre of several research efforts [35, 51,52,53,54,55,56,57,58,59,60]. Among the commercial COC, norbornene (or Bicyclo[2.2.1]hept-2-ene), 1, 3-dicyclopentadiene and 5-vinyl-2-norbornene can be found as cyclic olefins.
Norbornene can be homo- or copolymerized via two different pathways, ring-opening metathesis polymerization (ROMP) and vinyl addition polymerization.
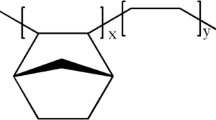
Ethylene–norbornene copolymers (ENCs) can be obtained by the first synthesis pathway given in Fig. 1: the first mechanism corresponds to the vinyl polymerization of a cyclic monomer (norbornene or 8,9,10-trinorborn-2-ene) and ethane, giving rise to COC. The vinyl-type ENC is a 2,3-connected rotationally strongly constrained cyclic olefin copolymer. It gives rise to high thermal stability copolymers, having high transition temperatures, excellent optical transparency and low birefringence. This synthesis pathway was developed by Mitsui under the trade name Apel® and was developed by Ticona Celanese until 2006 under the trade name Topas®. Since then, the production of Topas COC has been sold to two Japanese companies, Daicel and Polyplastics, who merged into a new entity called Topas Advanced Polymers [1, 61, 62].
The second pathway deals with the ring-opening metathesis polymerization (ROMP) of various cyclic monomers, followed by hydrogenation, and gives rise to COP [63,64,65]. Those copolymers are unsaturated and generally exhibit good solubility in various solvents. This pathway is developed under trade names Zeonex® and Zeonor® (Zeon Chemical) for norbornene and C-Z Resins (Daikyo) as cyclic monomers [66, 67].
Figure 1 illustrates those two synthesis pathways.
Structure and properties of ethylene–norbornene copolymer (ENC)
The chemical structures of the ethylene–norbornene copolymers (ENCs) have been investigated by several researchers [39, 68,69,70,71,72,73,74,75]. They used different analytical techniques such as DSC, NMR, SEC, refractometry and viscometry to show the correlation between the chemical structure of ENCs and their physical properties. In a series of papers, Ruchatz et al. as well as Tritto et al. [52,53,54, 60, 76, 77] evidenced the relationship that exists between catalyst and polymer structures. It has been widely discussed in the literature that the microstructure of the ENCs depends on the nature and geometry of the catalyst used in the synthesis [52,53,54, 56, 57, 60, 72, 78, 79]. Changing the catalyst system can give copolymers with different properties, depending on the ability of metallocene to form blocks of cyclic monomer units and different stereoregularities within the norbornene blocks [42].
Three different microstructures have been highlighted and are shown in Fig. 2.

Reprinted from [80] with permission from Wiley-VCH Verlag GmbH & Co. KGaA
Different sequences of ethylene–norbornene copolymers (E ethylene; N norbornene).
The copolymerization of ethylene and norbornene can produce copolymers with various types of sequences: alternate sequences (NENEN) or random sequences in blocks (isolated: EENEE, mesodyads: ENNE or longer blocks: ENNNE). The alternating copolymer is partially crystalline and shows melting points up to 320 °C and also the lowest glassy state temperature (T g).
The crystallinity of COC is influenced by the microstructure. It decreases and then disappears when the rate of norbornene in the copolymer increases. Indeed, the copolymer becomes amorphous at a norbornene level greater than 14 mol% [81,82,83]. The crystallinity also depends on the catalyst structure: vinylic polynorbornene, prepared with zirconocenes, are partially crystalline and therefore insoluble, whereas soluble amorphous polynorbornene are obtained with late transition metals [84].
13C-NMR investigations of the microstructure of ENCs supplied by Hoechst Celanese with molar percentages of norbornene varying from 35.6 to 79.0, were recently reported by Rische et al. [71]. The authors showed by DSC, NRM and WAXD (wide angle X-ray diffraction) that there are two distinct structure patterns depending on the norbornene content: ENCs with a norbornene molar fraction of less than 50% mainly consist of both blocks of alternating norbornene/ethylene sequences and polyethylene sequences, whereas copolymers with a norbornene molar fraction higher than 50% show a more random structure with different structural features.
Wilson et al. [85], Forsyth et al. [80, 86] and Scrivani et al. [87] worked on laboratory-made ENCs, whereas Makrocka et al. [88, 89] worked essentially on Topas COC (8007 and 6013). They all indicated by means of dynamic mechanical thermal analysis (DMTA) and DSC the presence of 3 motional processes related to dynamic glass transition assigned to the different segments of the ENCs. Some authors reported a linear relationship between norbornene (NB) and glass-transition temperature (T g), while others showed a deviation from linearity.
Ruchatz et Fink [54] established the following linear relationship:
Scrivani et al. [87] showed that the efficiency of the catalyst system guarantees, among the different copolymers, a similar norbornene distribution along the chain. With the increase of NB contents, the authors induced an increase of dyads and blocks of three or more NB units in the polymer backbone. The inclusion of rigid norbornene units in the polymer chain, associated with the increase of T g, leads to considerable higher Young moduli and microhardness values in these COC.
Similarly, using differential refractometry, Brauer et al. [40] established a linear correlation between ethylene content and the glass-transition temperature as follows:
In contrast, some authors [80, 90] showed that copolymers with similar NB contents had significantly different T g. They showed that the stereospecific alternating copolymers were partially crystalline with T g around 30 °C lower than their random content parts. They have then stated that the glass-transition temperature depends more on the microstructure rather than solely on the NB content and that the stereoregularity of the chain segments affects copolymer’s T g.
In the case of COC and COP commercial grades, deflection temperature (HDT = heat deflection temperature) is often given. This temperature depends on the level of norbornene in the structure which influences the glassy temperature T g [80]. Indeed, the grades that have the highest levels of norbornene have the highest T g and are more resistant to temperature. Nomenclature grades of Topas® consist of four numbers; the first two represent the intrinsic viscosity and the last two refer to the deflection temperature.
Table 1 lists the major grades of Topas®, Zeonex®, Zeonor® and Apel™ with a brief description of their properties. Some other grades of Topas® are available for specific applications in the field of optics, medical or diagnosis but are not cited in scientific literature.
Specific chemical structures of cyclic olefin copolymers also give a combination of properties that are listed in Table 2 with their various applications.
The thermal behaviour of three series of mono- and bicyclic COC supplied by Hoechst Celanese, Mitsui Chemical and Nippon Zeon Co was studied by Liu et al. [91] using thermogravimetric analysis under nitrogen atmosphere. Authors focused on the influence of the chemical composition and microstructure on the degradation temperatures and kinetic parameters. In this study, cyclic structure content varied from 20 to 60 mol% and T g from 80 to 177 °C. High-density polyethylene (HDPE) was used as a reference. COC have a superior thermal stability on polyolefin materials but depend on COC’s microstructures due to steric effect, chain stiffness of cyclic structures and branching effects. Kinetic parameters, such as Ea, the activation energy and A, the pre-exponential factor, were also calculated, and no correlation between those values and the cyclic structure content of COC could be evidenced.
Ekizoglou et al. [92] also studied the thermal properties of laboratory-made ENCs, having a norbornene molar fraction varying from 0.45 to 0.47. They showed that those ENCs had, respectively, T g varying from 108 to 123 °C (from DSC measurements) or from 132 to 143 °C (from DMTA measurements). They are the only authors, to our knowledge, who take into account the fact that the molecular weight of the copolymer is very important for the intrinsic values of T g. They showed that T g of ENCs of the same norbornene content, increases significantly up to 6–10 × 104 g/mol and that above, T g is almost constant.
Dorigato et al. [93] studied the behaviour of melt compound linear low-density polyethylene (LLDPE) with Topas COC (8007) by means of DSC, TGA and DMTA. They showed that the incorporation of COC segments in LLDPE enhanced the thermo-oxidative degradation stability of LLDPE (thanks to the resulting limitation in oxygen diffusion through the sample) and that it also enhanced the elastic modulus and decreased strain at breaking point.
In order to test the high thermal resistance as well as COC moulding feasibility, Liu. et al. [94] synthesized COC with a high glass-transition temperature (T g > 200 °C) in the presence of metallocene as catalysts. A discoloration of this laboratory-made COC took place after pelletization by extrusion moulding owing to thermal oxidation and production of alkene groups. The authors successfully added several antioxidants (Irganox 1010, Irgafos 168, Irganox HP2225 and Irganox HP2921) to prevent thermal oxidation and colour staining. It was shown that adding 1 wt% of antioxidant in laboratory-made COC eliminated discoloration and allowed the COC to be used for optical applications. They also added three antioxidants (Irganox 1010, 1330 and 1024) into mCOC with high T g in another study to prevent the thermal oxidation and colour stain [95]. Using DSC measurement under oxygen, they demonstrated that oxidation induction temperature increased with the presence of antioxidant, showing the protective effect of additives.
Additives added to ENCs
In order to protect the material from oxidation or photochemical reactions and in order to improve processing, antioxidants and lubricants are added to the polymer bulk. Antioxidants are present at low concentrations (typically less than 0.3% in mass) as required by the health regularity authorities such as the European Pharmacopeia. Qualitative and quantitative analyses are then necessary to understand further degradation processes and assess the quality of the plastic material in question. Table 3 gives the list of antioxidants and lubricants added to ENCs and referenced in the literature reviewed in the present paper. We should notice that most of the papers do not consider the additives added although it is important to have this information when considering polymer ageing.
Ageing
Several ageing conditions exist, and among them, temperature under inert conditions, thermal oxidation, photodegradation and radiation are the most cited. An exhaustive review of the weathering of polymers has been written by White et al. [103] that shows the complexities of the various degradation processes and compares the accelerating laboratory tests with outdoor service behaviour, especially on PVC, PE, PP, PC and PS. In the literature, we essentially found studies made on Topas® (COC) of different grades (5013, 6013, 6015, 6017, 8007), on Apel™ (COC) (6509, 8008) and on Zeonor® and Zeonex® (COP) (750R). Some grades contain additives such as phenolic antioxidants and lubricants (see Table 3). Antioxidants play a role during material ageing, and all additives undergo degradation during ageing. We shall now examine what can be found in the literature dealing with ageing by thermal oxidation, photodegradation by UV rays and by ionizing radiation (plasma, gamma rays and electron beam).
Like all types of polymers, the behaviour of ENCs as they age, depends on several factors and namely, the environment, the ENC grade in question (norbornene content rate and additives concentration), irradiation dose and the presence of oxygen in the surrounding medium. The main chemical changes that may occur during ageing are:
-
Competition between chains scissions and cross-linking,
-
Formation of gas and low molecular weight compounds (LMWC),
-
Formation of double bonds,
-
Under oxygen, oxidation groups are created.
Ageing by thermal oxidation
This type of ageing has been referenced by several authors [91,92,93,94,95, 104,105,106]. Thermal ageing is performed to study the thermal stability and the thermal properties of materials. ENC commercial grades described in ageing studies are Topas® 5013, 6013, 8007; Apel® 6509 and 8008, some of which contain additives. Ageing by thermal oxidation is done using thermal techniques such as DSC or TGA under air or nitrogen.
Yang et al. [106] used FTIR microscopy and thermogravimetry under air atmosphere to study the kinetic behaviour of the thermal oxidation of Topas® 5013. In this study, authors mixed TiO2 powder with ENCs to improve the thermophysical and dielectrics properties of electro-optic devices, and this material was compared with pure metallocene COC (mCOC). Figure 3 shows the evolution of mCOC thermal oxidation. Thermogravimetry is coupled with FTIR spectrometry, and specific bands are followed during oxidation: 2953 cm−1 corresponding to the elongation vibration of C–H, bands at 1779 and 1726 cm−1 corresponding to the elongation vibration of the (COO) and (C=O), attributed to the formation of lactones and ketones, respectively. The absorbance band at 1602 cm−1 is the vinyl stretching vibration.

Reprinted from [106] with permission from Elsevier
Evolutions of infrared peaks of pure mCOC during the thermal oxidation.
These different bands show the degradation of the copolymer during ageing. For heating temperatures ranging from 120 to 550 °C under air atmosphere, a decrease in the IR absorbance band at 2953 cm−1 was observed from 250 to 400 °C and attributed to dehydrogenation. Oxidation of the copolymer is initiated at 270 °C, evidenced by the increase of the elongation vibration of (C=O) at 1726 cm−1. An increase is observed in both the intensities of lactones (1777 cm−1) and vinyl bonds (1601 cm−1), associated with the decrease of ketone groups at 360 °C. Over 520 °C, all bands disappear, due to the decomposition of the material. Two-step degradation process is shown where 80% weight loss occurs between 415 and 465 °C, corresponding to polymer chain scissions and oxidation once the dehydrogenation is complete, and over 465 °C, the last 20% weight loss corresponds to the degradation of the polymer. Authors noted that in the presence of TiO2, a thermal stabilization of the polymer is observed which delays the occurrence of major cracking from 454 to 473 °C.
The thermo-oxidative behaviour of two series of ENCs ((Topas (6013 and 8007) and Apel (6509 and 8008)) containing Fe, Co and Mn stearates as prodegradant additives, was investigated by Gutiérrez-Villarreal et al. [105]. The percentage of norbornene unit in Topas is of 54 and 38 mol%, whereas it is about 20 mol% in Apel’s COC. The incorporation of transition metal salts as prodegradant agents during the processing had been tested before on polyolefin [107, 108]. Authors used an air-circulating oven at 70 °C for 150 days. The progress of degradation under these conditions was analysed by FTIR, mechanical and thermal techniques.
Authors used the Carbonyl Index (CI) to characterize the degree of degradation of cyclic olefins, which was defined as a ratio of an absorbance carbonyl around 1712 cm−1 (a by-product of the thermal oxidation) and the absorption band at 1456 cm−1 (CH2 rocking mode peak) as follows [105]:
Even though the authors showed differences in oxidation between Topas and Apel grades, they noted that both copolymers are stable from a thermal oxidative perspective. They demonstrated that Topas COC have higher CI than Apel COC, which is explained by the fact that Topas is more susceptible to thermal degradation because of their higher percentage of norbornene units, which induces a higher tertiary carbon content which are weak sites for oxidation. Amorphous regions are also more frequent when NB content increases, which affects the oxygen diffusion, leading to easier oxidation and chain scissions. Topas films are harder and stiffer due to their high content of NB unit.
Our team also studied the thermo-oxidative stability of electron beam-irradiated ENC (Topas 8007) [104]. We used a series of commonly used thermal methods (TGA, DSC, OIT measurements) to evaluate polymer matrix oxidation level. The comparison of ENC degradation under O2 and N2 observed using TGA showed that the copolymer is degraded at lower temperature under O2 compared to N2 conditions, with the formation of several oxidative species (cf Fig. 4). Hydroperoxides and peroxy radicals are certainly formed around 225 °C under O2. At degradation temperatures above 300 °C, both polymer and antioxidant undergo several successive degradation steps at lower temperatures than under N2 where only one step is evidenced around 400 °C.

Reprinted from [104] with permission from Elsevier
Comparison of TGA curves under nitrogen and oxygen of a 8007S04 sample (0_Add) and of Irganox 1010®.
We also observed the protective effect of the antioxidant (Irganox 1010) against oxidation of the copolymer. Indeed, the measurement of induction temperature times by DSC showed the importance of the antioxidant in delaying the oxidation of the polymer.
Ageing by photodegradation under UV radiation
UV radiation ranging from 200 to 400 nm represents only 6% of the total intensity of the solar spectrum on the surface of the earth. However, the energy of radiation in this range is of the same order of magnitude as the energy of a chemical bond. Photodegradation induced by these rays may occur in the absence of oxygen (inducing chain breaking and/or cross-linking) and in the presence of oxygen (oxidative photodegradation) and furthermore may be accelerated at high temperatures [109].
Few papers were found, dealing with the photomodification of ENCs under UV rays:
UV radiation-induced photodegradation of three ENCs (from Polyplastics Co, Japan) with various norbornene content (50, 52 and 57 mol%) and similar stereoregularity, has been studied by Nakade et al. [110]. Photo-oxidation was induced with a Xenon arc lamp (1500 W—63 °C) and evidenced—by means of weight measurements after solubilization in chloroform—both scissions (soluble part) and cross-linking (insoluble part). The soluble part was then analysed by FTIR, 1H NMR and SEC. Among the modifications observed after photoageing, formyl, acyl, hydroxyl, formate groups and double carbon–carbon bond were highlighted. Authors also showed that photodegradation increased with increasing norbornene content.
Nakade et al. [110] proposed three formation pathways of photodegradation products in ENCs (Fig. 5).

Reprinted from [110], with permission from Elsevier
Formation pathways of the photodegradation products. a Formation pathways of the radical species, b formation pathways of acyl and formyl groups, c formation pathways of the double bonds.
-
(A)
Formation pathways of the radical species: when ENCs are irradiated with UV radiation, both the norbornene ring and ethylene bond are excited. The excitation energy is then transferred to the nearest C-H bond, resulting in the cleavage of this bond and the release of hydrogen, leading to polymer radical formation. The polymer radical can react with molecular oxygen, to produce polymer alkoxy (PO°) and hydroxyl (OH°) radicals.
-
(B)
Formation pathways of acyl and formyl groups: the polymer alkoxy radical (PO°) and/or hydroperoxide (POOH) may also cause chain degradation by β-scission, with the formation of formyl and acyl groups.
-
(C)
Formation pathways of double bonds: photodecomposition of hydroperoxide groups with the formation of double bonds, hydroxyl functions and ketone groups in polymer.
Pilar et al. [102, 111] also worked on the photo-oxidation of several polymer materials induced by a Xenon light (Weather-Ometer (WOM), producing special irradiance 0.5 W m−2 nm−1 at 340 nm, 60 °C, 20% RH) for 200 days. They compared 6-mm-thick plaques of polypropylene, high-density polyethylene, polystyrene and Topas 8007 stabilized with different hindered amine stabilizers (Tinuvin 770, Flamestab, Chimassorb). They used attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and evidenced the presence of (OH) vibrations due to carboxylic acid at about 3350 cm−1 and carbonyl bands as well as vinyl bands in the 1850- to 1550-cm−1 spectral range. Three main bands of lactone (1780 cm−1), vinyl (1650 cm−1) and combination of ester and carbonyl (1720 and 1740 cm−1) were found by deconvolution (Fig. 6). For the non-stabilized samples, a higher concentration of oxidation products was found in COC than in PP. Nevertheless, the same vibration bands were observed with, in both cases, the predominance of the combination bands located at 1720–1740 cm−1.

Reprinted from [102], with permission from Elsevier
Distribution of oxidation products determined by deconvolution of the carbonyl and vinyl regions in the ATR-FTIR spectra of the non-stabilized COC plaques after 144 days of WOM exposure.
Also for the purpose of inducing surface modifications, Jena et al. [112, 113] used UV light on Topas COC (8007 and 6015) in order to enhance surface properties for microfluidic chips production. They experimented with the photografting of acrylic acid, 2-hydroxyethyl acrylate (HEA) and 2-methacryloyloxyethyl phosphorylcholine on the COC’s surface in order to minimize the adsorption of analytes and reduce the adhesion of cells. They used UV irradiation from an UV lamp at 265 and 365 nm and irradiation intensity of 130 mW cm−2. The best results were obtained with acrylic acid grafting. Contact angle measurements as well as FTIR, XPS and SEM showed that a stable hydrophilic surface could be obtained. The surface wettability is an important aspect in that it improves biocompatibility for the microchannel in microfluidic devices. They succeeded in improving the surface hydrophilicity as well as resistance to both protein adsorption and cell adhesion that are of great importance for the development of microfluidics in bioanalysis.
Tsao et al. [23] used UV/ozone surface treatment for inducing surface modification of COC (Zeonor 1020) in microfluidic systems. UV/O3 treatment is a technique initially developed for the removal of organic contaminants from semiconductor substrates, but at sufficiently high energy levels, UV/O3 exposure can break polymer chains and insert oxygen-containing functional groups into the surface. Surface modification (UV/O3—14 mW cm−2 at 254 nm) is followed by contact angle measurements in water. A decrease of contact angle with exposure time (95° to 43°) was observed, which indicates the increase of surface hydrophobicity. The authors also noticed that COC surfaces remained relatively stable compared to PMMA when exposed to solvents (acid, basic and neutral). Hydrophobicity and solvent stability are important parameters for microfluidic substrates because fluids must easily flow through them.
Ageing by ionizing radiation
There are several types of ageing process involving radiation (ionizing radiation such as plasma treatment, gamma and electron beam radiation and even swift heavy ions). ENCs can undergo plasma, gamma or electron beam treatment in order to be sterilized, but most of the time a surface modification is desired [11, 20, 96, 97, 100, 114,115,116,117,118,119,120].
The irradiation of polymeric materials with ionizing radiation leads to the formation of reactive intermediates, free radicals, ions and atoms in excited states. These intermediates can follow several reaction pathways that result in disproportionation, hydrogen abstraction, rearrangements and/or the formation of new bonds; all those reactions lead to the subsequent alteration of the material structure via radical chemical action. The degree of transformation depends on the structure of the polymer and on the treatment of the polymer before, during and after irradiation [121, 122]. Oxidative scission is initiated by a reaction between an oxygen molecule and a free radical, which leads to the formation of a peroxyl radical (POO°). This radical in turn undergoes other hydrogen abstraction reactions and combinations that leads to polymer degradation and formation of numerous oxidation products: hydroperoxides (POOH), peroxides (POOP), aldehydes, esters, ketones, carboxylic acids and alcohols [123].
Plasma treatment of COC
Plasma treatment has been used, for surface modification for years. In the case of ENCs, plasma treatment is the most described radiation treatment in the literature. The advantage of plasma treatment is that it only modifies the outermost layer in the range of few nanometres [124] and promotes the wetting and the adhesion properties of the material in question. In the case of polymer plasma treatment, the main factors responsible for the surface effects are: free radicals present in the plasma, vacuum UV radiation in the chamber and impingement of ions on the surface.
With plasmas derived from simple gases (oxygen, nitrogen, noble gases, etc.) the major modifications can be described as: ablation or etching (that induces an increase of the roughness), degradation of polymer molecules (that give rise to leachables), cross-linking or branching of near-surface molecules and introduction of new functional groups. These four effects occur concurrently, and depending on the processing conditions and reactor design, one or more of them may predominate. In most cases, these processes affect only the top few molecular layers (about 100 Å).
Johansson et al. [125] studied the effects of low-pressure radiofrequency air plasma treatments on six different polymers (Topas 5513X2, Zeonex 480R, PMMA, SAN, PS and PC) in order to hydrophilize their surfaces and to provide good cell culture properties with stable surfaces even after ethanol washing. Nevertheless, one of the drawbacks of surface treatments is the formation of low molecular weight oxidized material (LMWOM) on the surface due to polymer molecule degradation. The LMWOM compounds might then be solubilized by washing (inducing leaching) and might also change the physico-chemical properties of the polymer surface. Air plasma treatments affect both the wettability and the elemental surface composition of all materials. The introduction of oxygen-containing groups in polymer surfaces induces a high decrease in contact angle values (around 40°–60°) even at low power/flow ratios. Functions created after plasma treatment and evidenced by XPS are hydroxyls, ethers, epoxides and carboxylic acids. In this study, the authors showed that PMMA is less affected by air plasma than the other polymers and they attributed this result to the depolymerization mechanism of PMMA during plasma treatment. The authors also demonstrated that Zeonex 480R (COP) and Topas 5513X2 (COC), although having similar compositions, give very different XPS spectra after plasma treatment. Moreover, they showed that only Zeonex, PS and SAN gave good cell growth properties after plasma treatment, whereas it was not the case with Topas and PMMA. They attributed this difference to the different additives present in the polymers.
In an another field of application, that is the adhesion to metal, Nikolova et al. [126] came to the same conclusion that LMWOM and additives concentration at the surface of polymer could play a role in the plasma-induced surface modification. They studied Topas 6017’s adhesion to Al and Cu. Similarly, Hwang et al. [116] compared the adhesive potential between a coating layer (indium tin oxide, ITO) and a COC (Apex, Mitsui Chemicals) after oxygen plasma treatment. They used specific surface analysis techniques (contact angle measurements, XPS and AFM) to examine the surface modifications. They concluded that adhesive performance could be improved by increasing interactions between the acceptors (coming from the negative-charged oxygen ion of the hydrophilic groups) and the donors (coming from the positive-charged metal).
Finally, in medical applications, the treatment of osteochondral defects was reported by Petrtyl et al. [34] and Polanska et al. [127]. They used COC and COC/LLDPE blend, modified by low-pressure nitrogen plasma as polymer implants. The plasma treatment induced the formation of hydroxyl, carbonyl, carboxyl and amine functional groups at the surface, and several cell cultures demonstrated the suitability of those materials for tissue engineering.
Roy et al. [11, 20] used radiofrequency (RF) argon and argon–oxygen plasma to enhance COC (Topas 6015 and 5013) surface properties for applications in microfluidic devices. The goal was to investigate the influence of ageing on the surface properties of plasma-treated COC. Plasma treatment induced photo-oxidation through the reaction of trapped radicals with oxygen; authors reported the presence of chains scissions but also cross-linking and increase of the surface roughness (which may be linked to the removal of LMWOM). Roy proposed possible reaction pathways due to the plasma’s effect, without giving any proof of the nature of the radicals. To go further and to our knowledge, no paper dealing with ENC radicals can be found in the literature. ENCs are often compared to PE, and many papers can be found that deal with the nature of radicals created in PE, especially after RF plasma treatment [128, 129]. Recently, Mix et al. [130] compared the effect of RF oxygen plasma on linear, branched and hyperbranched PE and ENCs (Sumitomo chemicals, Japan—norbornene rate of 65%), considered as a sterically hindered copolymer. After washing the surface with THF and water, in order to dissolve LMWOM from the treated surface, XPS and DSC were performed. Plasma is known for inducing cross-linking on the surface of plasma-treated PE, and in this paper, the authors used electron spin resonance spectroscopy (ESR) to identify the formation of tertiary carbon inducing branching and cross-linking in the polymer chains. Unfortunately, the paper focuses on PE and no ESR results are given for ENCs. They showed that linear structures, having the highest tertiary carbon content, underwent more cross-links after oxygen plasma than branched polymers.
In the field of nanosciences, nanoimprint lithography (NIL) was developed with COC (Zeonex) and PMMA by Kettner et al. [131]. The polymers were pretreated under different plasma conditions in a plasma chamber using different gases with oxidizing (oxygen, nitrogen), reducing (hydrogen/helium) or inert (argon) properties. The goal of plasma treatment was to activate the bonding at low temperatures (below the glass-transition temperature of the polymers), in order to ensure that the structures were not deformed. The results demonstrated that the oxidizing gases had the biggest impact on the surface activation. Plasma pressure has more impact on the COC materials under study (Zeonex) compared to PMMA, where no correlation between the plasma pressure and the bond strength was measured.
Gamma radiation
Gamma radiation can be used for sterilization purposes and is appreciated for its high penetration and negligible heat production [132]. The accepted regulatory average dose for sterilization is of 25 kGy [133]. Radiation induces chemical reactions on polymer chains and modifies their properties because of chain scissions, chain branching and cross-linking. Usually, all these processes coexist but the predominant effect depends on several factors, such as the chemical structure and morphology of the polymer, the irradiation itself and the post-irradiation treatment. To our knowledge, only one team published studies on the effect of gamma rays on COC materials.
Kačarević et al. [134] studied the structural alternations of gamma-irradiated ethylene–norbornene copolymer (Topas 6015S-04 containing Irganox 1010 at 0.5 wt%). Samples were irradiated at 100 and 200 kGy by γ-rays from a Co60 source at a dose rate of 6 kGy/h and annealed at 60 °C for 2 h in vacuum before exposure to air. Oxidation products were evidenced by FTIR with in particular the formation of ketones (1718 cm−1) and aldehydes groups (1733 cm−1) in the amorphous zones. Gamma rays are known to induce oxidation when the irradiation is performed in air because of the low dose rate of gamma rays, compared to electron beams. The authors focused on the UV spectral modification because ENCs are used for their optical properties and modification of the transmittance parameter would be unacceptable for optical applications. They showed that changes in UV spectra occurred both in samples irradiated in air or water and correspond to the formation of double bonds at around 250 nm. The presence of antioxidant changes the shape of the spectrum, but modification still occurred. The authors also checked for modifications due to gamma radiation by measuring the glass temperature transition (T g) and showed that it increased for doses over 200 kGy, indicating that cross-linking occurred at that dose.
Further studies were conducted by Šećerov et al. [100] with the same polymer and irradiation conditions but using higher dose range (0–500 kGy) in the presence of air and in water. Gel content (insoluble fraction formed in copolymer irradiated) was measured by weighing the dry gel after extraction and was about 23% for the dose of 500 kGy in water (less when the irradiation was performed in air or with the presence of antioxidant). Cross-linking was then highlighted and again correlated with the T g increase with dose.
Electron beam irradiation
Electron beam irradiation has been used to sterilize medical goods for well over 50 years [119, 135, 136]. Because of its convenience and low cost, it provides an interesting and preferable alternative sterilization method compared to high-temperature steam autoclaving or dry heat that can damage materials, induce degradation and lead to losses of both shape and mechanical properties. Treatment with gamma or electron radiation has become a common process for the sterilization of packages. As in the case with gamma rays, electron beam irradiation induces chain scissions, cross-linking, oxidation and can also be used for chemical grafting [121, 122, 137]. It can also modify the mechanical properties as well as the surface properties of the polymer [138].
The behaviour of COC in response to accelerated electrons is an important issue, but only a few papers can be found, corresponding to the work of two research teams: our team [96, 97, 114] and Cerrada’s [115]. In those studies, Topas 6013 and 8007 as well as Zeonor 750R were irradiated with an electron beam of 10 MeV under air and ambient temperature. Doses applied varied from 25 to 233 kGy, depending on the authors.
The evolution of T g with irradiation was studied by Cerrada [115] who found that T g increased significantly with dose, indicating reduced mobility due to branching and cross-linking.
We studied additive degradation and modification of the polymer’s thermomechanical properties, such as elasticity, glass-transition temperature and swelling capabilities [96]. The insoluble fraction (forming a gel) of irradiated polymer chains was investigated in toluene for 24 h, and it was shown that only Topas 8007 could give an insoluble fraction of 35% after an irradiation dose of 150 kGy, whereas neither Topas 6013 [115] nor Zeonex 850 underwent gel formation in the same conditions. We also studied changes in molecular mass using SEC in different solvents (TCB at 135 °C and toluene at 60 °C), and the same behaviour was observed: for low radiation doses, the polymer underwent chain scissions, and over 75 kGy, cross-linking and branching were observed. We noticed that the effect of the radiation was less important in Zeonor 750 R than in Topas 8007 and made a correlation with the antioxidant concentration which is almost 3 times higher in Zeonor 750 R than in Topas 8007. Indeed, it has already been shown using polyolefins that the presence and the nature of antioxidant influenced the polymer degradation in terms of gel formation and cross-linking [139,140,141]. We showed that Irganox 1010’s concentration decreased with the increase of the dose, inducing the formation of numerous oxidation products. Using DSC studies done under oxygen atmosphere, we showed that the effect of the ionizing radiation is to lower the thermo-oxidative stability of the copolymer [104]. Indeed, the irradiation degrades the antioxidant and oxidation of the polymer is then facilitated. Nevertheless, oxidation induction time after irradiation is higher than for samples without antioxidant. During radiation, ENCs undergo both cross-linking and chains scissions; cross-linking might protect the polymer from oxidation by hindering O2 diffusion, but at the same time chain scissions generate degradation products that, once linked to the polymer chain, could also give antioxidant properties. We demonstrated [97] the generation of oxidized species (LMWOM) by using both SEC and RP-HPLC. Those studies revealed that new species appear after radiation, corresponding to both polymer chain scissions and degradation and recombination products of Irganox 1010® (Fig. 7). Low molecular weight compounds merged from radiolysis, even at a dose of 25 kGy. Moreover, as additives are added to the polymer, they also undergo degradation during ageing, a factor that is often neglected. To conclude with our observations, we noticed that post-irradiation oxidation of ENCs, greater on the surface than in the volume, depends primarily on the grade of the ENC being analysed, its content of norbornene or presence of lubricant, ageing conditions and irradiation dose.

Reprinted from [97], with permission from Elsevier
HPLC chromatograms of Topas® 8007 D-61 extractables for non-irradiated sample and 25-, 75- and 150-kGy irradiated samples. The detection was performed by a diode arrays set at wavelengths a 280 nm and b 250 nm.
Finally, using XPS and AFM, we showed that the roughness and the wettability of the surface were enhanced by electron beam treatment even for a low dose (25 kGy) [114]. As these materials can be used as medical devices or pharmaceutical packaging, surface modifications must be considered. Figure 8 evidences the effect of a 150-kGy dose on the amplitude and organization of the roughness of the surface. Depending on the size of the particule in interaction with the polymer, the roughness measured here may or not be significant; for example, if a bacteria (size around 1 µm) is able to fit the asperities of the irradiated spin-coated film, this texture will enhance its adhesion by increasing binding potential. But, for a smaller entity like a chemical molecule (size around 1 nm), the binding potential will not be increased, but a higher number of entities might adsorb because of the increase in the surface area.

Reprinted from. [114], with permission from Elsevier
3D images of AFM topographic images (2 µm × 2 µm) of a non-irradiated spin-coated films, b 150-kGy irradiated spin-coated films, c non-irradiated extruded films and d 150-kGy irradiated extruded films.
In this study, the interaction of irradiated ENCs with three drugs, at concentrations close to that of pharmaceutical formulations (benzalkonium chloride, phenylephrine and isoprenaline), was studied. We showed that the drugs sorption, which was not detectable on the non-irradiated ENCs, was not enhanced by the surface changes.
Ion beams
Ion beams can be used for surface modification of polymer materials, because of the limited penetration depth of the ions. In general, this technique induces various improvements to the mechanical, tribological, optical and electrical properties of polymers [142] but may also lead to ionization, displacing atoms, sputtering, carbonization, production of free radicals, which induce cross-linking and chains scission that gradually and continuously contribute to modify or degrade the properties of polymer [143]. In the literature, we only found two papers, written by the same research group, dealing with the modification of an ENC (Topas 6015S-04) by ion beams (C4+ and N4+) [118, 120].
Kačarević et al. [118] studied the modification of ENCs with respect to their thermal stability. They reported that Topas 6015 has good stability to radiation through measured heat and thermal properties and that it was stable up to 420 °C with degradation maximized around 480 °C. The authors showed a slight increase in thermal resistance with the increase in ion fluence. They attributed this result to small changes in local chain architecture that can affect both the thermodynamic and dynamic properties of the melt. They also evidenced a decrease in resistivity with an increasing ion fluence, due to formation of conjugated double bonds in copolymer macromolecular chains. By means of UV–Visible spectroscopy, they showed that π–π* electronic transitions increased with ion fluence, due to the formation of unsaturated bonds, as already mentioned in polyimide [144]. Šiljegović et al. [120] wanted to characterize the optical changes of Topas 6015 induced by radiation, for applications in the fields of optical sensors and light-emitting diodes. They showed that optical energy gap and optical activation energy decreased with increasing fluence of ions, an effect that is attributed to the formation of conjugated double bonds. The Raman spectroscopy also showed that the number of carbon atoms in carbon clusters was found to increase with increasing the ions fluence, while AFM analysis revealed that the surface roughness gradually increased too.
Degradation of the additives
During polymer processing, additives such as antioxidants, anti-UV agents or lubricants are added in order to protect the polymer against the ageing effects of manufacturing, sterilization and storage. Our research team and other groups studied the degradation of polymer additives under ionizing radiation during the sterilization of plastic materials [145,146,147] and their degradation during storage [96, 99, 148]. Degradation products are formed and are able to diffuse from the polymer matrix into the surrounding environment [149,150,151]. This can occur in polymer applications used for drugs, biological media or even food products. It then becomes a challenge to identify those leachables and extractables and to study their toxicity. We studied, for example, the fate of phenolic antioxidant Irganox® 1010 present in Topas 8007 and showed that the antioxidant concentration decreased as the irradiation dose increased [97]. This decrease is explained by the degradation of the antioxidant and/or by the formation of covalent bonds between the degraded molecules and the polymer chains. Allen et al. [152] have already reported similar bonding by 14C-labelling of the antioxidant. The authors showed that Irganox 1076 gave one significant degradation product, whereas Irganox 1010 gave three major and several minor degradation products after gamma radiation for doses up to 50 kGy. In a second study, after electron beam irradiation of Irganox 1330, the authors used LC–MS to describe the presence of a wide range of products (and among them, quinones) similar to those observed after chemical oxidation [153]. They also compared radiation ageing and thermal ageing of different phenolic antioxidants [154]. They observed that Irganox 1010’s degradation was different under radiation and under thermal oxidation, whereas Irganox 1330 and 1076’s degradation was independent of the ageing process.
For the highest molecular mass additives such as Irganox 1010 (MW > 1000 mol g−1), other authors [155,156,157,158] tested ionization suitability using different desorption ionization techniques and highlighted that in the case of additives mixtures, the difference in ionization of the components must be taken into account.
Study of interaction between COC and contact environments
COC are known to be materials with good chemical inertness, which promoted their use in the pharmaceutical and medical fields. Nevertheless, as we emphasized earlier, when those polymers undergo ageing, LMWOM appear and can migrate out of the matrix and as a consequence, interactions between polymers and contact liquids can occur. Many publications deal with those interactions in terms of quality and safety. Jenke’s group [151, 159, 160] made a huge contribution to these studies. In order to validate the use of a polymer as pharmaceutical container, regulatory authorities require proof of the absence of interactions and analytical studies must be performed [151]. During the contact of the drug product and its packaging system, interaction can occur until its shelf-life expiry and among them, migration or leaching of chemical substances out of the packaging system into the drug product formulation. These chemical substances can potentially affect the quality of the drug product and may have an adverse effect on the patient [101]. In order to assess the safety of extractables in various dosage forms, the challenge of establishing a threshold for the identification and qualification of leachables was addressed for inhaled and nasal drug products. These authors recommend vigorous extraction techniques with multiple solvents of varying polarity and analysis techniques with different sensitivity and selectivity (GC for volatile extracts, LC/UV/MS for non-volatiles extracts).
Paskiet et al. [101, 160] studied the interactions between parental and ophthalmic drug products using five polymeric materials (polyvinyl chloride, brominated isobutylene–isoprene rubber, low-density polyethylene, polycarbonate and COC). In the case of COC, low potential leaching levels were identified [151]. In these studies, the nature of COC was not specified, but the authors indicated the presence of Irganox 1010 and Ultramarine blue in unspecified quantities. The predominant extracted metals are obtained in low pH extracts and include Ca, Na, Mg, Br, Fe, Zn and Al. The most important volatile compound was cis-decahydronaphthalene, and few identifiable organic extractables were obtained in aqueous pH 2.5 and pH 9.5 extracts.
In a previous paper, our research group used UV spectroscopy to study the sorption phenomenon of drugs (phenylephrine, benzalkonium and isoprenaline) after short storage times with electron beam-irradiated Topas films (8007) containing the antioxidant Irganox 1010 [114]. The sorption, which is not detectable on the Topas® 8007, was not enhanced by the surface changes. But it would be worth taking this study further by using extreme conditions for drug sorption storage and other adsorbing entities of greater size such as bacteria. In this case, roughness will certainly have a stronger impact because the size of the asperities created by treatment may increase the anchoring of the bacteria, which have a micrometric scale. This kind of studies is very important, because favouring the anchoring of bacteria on a medical device can increase the occurrence of nosocomial diseases.
Migrants from aged ENCs were evaluated comprehensively using SEC and HPLC, in one of our studies [114]. The profile of migrants obtained showed a distribution of molar mass ranged between 1000 and 500 g/mol. ENC oligomers are present among the migrants. They are hydrophobic in nature and favour sharing in less polar media. We highlighted these migration phenomena [99]. When these materials are immersed in polar solvents, sorption of the solvent into the polymer promotes swelling of the polymer chains and thus helps the mobility of migrants. More significant migration of phenolic antioxidants such as Irganox 1010® and BHT® can be observed in water during storage at elevated temperature 60 °C, even if these additives are insoluble in water [96].
Toxicity study of COC
From interaction studies, we learn that potential migrants can influence the quality of both materials and media in contact with them (pharmaceutical formulations, biological media). In addition to migrants, macroscopic and microscopic properties of materials used for the manufacturing of medical devices guide their interaction with the biological environments in question. In an ideal scheme, all the migrants should be identified precisely in order to verify their specific toxicity. Global toxicity can be evaluated using well-established methods. The in vitro cell culture-based methods represent the initial phase of biocompatibility testing of materials. They are performed by putting the materials and/or their extracts in contact with cells and subsequently investigate various cellular functions. A wide range of assays are reported in the literature. We can cite the morphological study which considers the cells morphology in contact with the material and particularly the cells’ adhesion to their surface, or indicators of the cellular morphology, such as lactate dehydrogenase, which is a cytosolic enzyme released by many different cells. Its analysis provides information on cellular membrane integrity [161]. Another very widely used assay for cytotoxicity screening [51] is the MTT test which measures the activity of the mitochondrial enzyme succinate dehydrogenase in active living cells through a colorimetric detection [162]. Reactive oxygen species (ROS) are studied and correspond to a group of chemically reactive ions, radicals and molecules derived from oxygen. They play an important role in cell signalling and can be generated in the course of normal aerobic metabolism or when the cells are exposed to a variety of stress conditions [163, 164]. High levels of ROS in cells in contact with a specific sample are an indication of oxidative stress induced by the sample.
Among the different types of cells used for biocompatibility assays, we find L929 fibroblasts, recommended in the international standard for the biological evaluation of medical devices, and human umbilical vein endothelial cells (HUVEC) for materials or substances in contact with the cardiovascular system. Dosage of cytokine production by macrophages in contact with materials can be investigated in order to evaluate the induced inflammatory reaction. Haemolysis, coagulation and platelet aggregation assays are generally performed to investigate the haemocompatibility of materials and their extractables.
In the case of COC, few studies are reported. Preliminary toxicity studies were made by Van Midwoud et al. [165]. These studies focused on the fabrication of biocompatible microfluidic devices from thermoplastics such as poly(methyl methacrylate) (PMMA), polystyrene (PS), polycarbonate (PC) and cyclic olefin copolymer (COC Topas). Biocompatibility was assessed by culturing human hepatoma cells (HepG2) on UV/ozone and oxygen plasma-treated surfaces. This comparison of the adsorption property and biocompatibility of different plastics indicated that only UV/ozone-treated PC and COC devices satisfied both criteria.
In a very recent study, our team performed a series of in vitro biocompatibility test (cell morphology, MTT, LDH, ROS) on different grades of ENC polymers in contact with different cellular lines, recommended by the standard ISO 10993 part-5 (in vitro cytotoxicity evaluation of medical devices). Topas® 8007 and 6015 with or without antioxidant and/or lubricant were included in this study, after being sterilized with electron beam at 25 kGy. Results showed the absence of any cytotoxicity signs in the cells after 48 h of contact with sterilization-aged materials (the date from this study is as yet unpublished).
Conclusion
This review concerns the physico-chemical ageing of plastic materials and in particular of ethylene–norbornene copolymers. At the molecular level, oxidation mechanisms and structural changes are the major modifications observed in ENCs after ageing.
Whatever the ageing process (temperature, UV or ionizing radiation), oxidation is mostly evidenced by FTIR and OIT measurements. The same chemical functions are shown to occur, namely (C=C) and (C=O) unsaturation bonds (vinyls, α-β-unsaturated ketones, esters, acids, ketones, lactones, etc.). But it is difficult to compare oxidation processes in a quantitative manner because not all the papers deal with the same ENC grades (in terms of norbornene levels and molecular weight) and composition (in terms of additives and additives concentration), when these data are provided. Nevertheless, oxidation is more pronounced when NB yield increases because amorphous regions become more frequent and these facilitate oxygen diffusion. In the case of radiation, the increase of T g with radiation dose, due to cross-linking, has been highlighted, but in most cases ageing causes chains scissions that lead to the creation of degradation compounds that exhibit high mobility, corresponding to their low molecular weight and/or high polarity. These LMWC can migrate in contact media (pharmaceutical formulations, biological liquids) and can affect patient safety. Hence, these migrants must be identified and their toxicity evaluated.
To our knowledge, no research has been performed to identify and quantify these low molar mass compounds. It would therefore be of great interest to study them to better understand the interactions that can take place between these materials and their environment.
References
Lamonte R, Mac Nally D (2000) Uses and processing of cyclic olefin copolymers. Plast Eng 56:51–55
Lamonte R, Mac Nally D (2001) Cyclo olefin copolymers. Adv Mater Proces 159(3):33–36
Khanarian G (2001) Optical properties of cyclic olefin copolymers. Opt Eng 40(6):1024–1029
Eakins MN (2005) New plastics for old vials. BioProcess Int 3:52–58
Shin JY, Park JY, Liu C, He J, Kim SC (2005) Chemical structure and physical properties of cyclic olefin copolymers. Int Union Pure Appl Chem 77(5):801–814
Woods EJ, Thirumala S (2011) Packaging considerations for biopreservation. Transfus Med Hemother 38(2):149–156
Mlejnek P (2007) Cycloolefin copolymers: processing, properties application. Univerzita Tomase Bati ve Zline—Fakulta technologicka, Zlín
Limam M, Tighzert L, Fricoteaux F, Bureau G (2005) Sorption of organic solvents by packaging materials: polyethylene terephthalate and TOPAS®. Polym Test 24(3):395–402
Eakins MN (2010) Plastic pre-fillable syringes and vials: progress towards a wider acceptance. Am Pharm Rev 13:12–16
Qadry SS, Roshdy TH, Char H, Del Terzo S, Tarantino R, Moschera J (2003) Evaluation of CZ-resin vials for packaging protein-based parenteral formulations. Int J Pharm 252(1–2):207–212
Roy S, Yue CY, Lam YC, Wang ZY, Hu H (2010) Surface analysis, hydrophilic enhancement, ageing behavior and flow in plasma modified cyclic olefin copolymer (COC)-based microfluidic devices. Sens Actuators B Chem 150(2):537–549
Hawkins WL, Worthington MA, Matreyek W (1960) Loss of antioxidants from polyethylene by evaporation and aqueous extraction. J Appl Polym Sci 3(9):277–281
Dillingham EO, Webb N, Lawrence WH, Autian J (1975) Biological evaluation of polymers I. Poly(methyl methacylate). J Biomed Mater Res 9(6):569–596
Kameoka J, Craighead HG, Zhang H, Henion J (2001) A polymeric microfluidic chip for CE/MS determination of small molecules. Anal Chem 73(9):1935–1941
Fredrickson CK, Xia Z, Das C, Ferguson R, Tavares FT, Fan ZH (2006) Effects of fabrication process parameters on the properties of cyclic olefin copolymer microfluidic devices. J Microelectromech Syst 15(5):1060–1068
Zhang J, Das C, Fan ZH (2008) Dynamic coating for protein separation in cyclic olefin copolymer microfluidic devices. Microfluid Nanofluid 5(3):327–335
Niles WD, Coassin PJ (2008) Cyclic olefin polymers: innovative materials for high-density multiwell plates. Assay Drug Dev Technol 6(4):577–590
Yi L, Xiaodong W, Fan Y (2008) Microfluidic chip made of COP (cyclo-olefin polymer) and comparion to PMMA (polymethylmethacrylate) microfluidic chip. J Mater Process Technol 208(1–3):63–69
Nunes PS, Ohlsson PD, Ordeig O, Kutter JP (2010) Cyclic olefin polymers: emerging materials for lab-on-a-chip applications. Microfluid Nanofluid 9(2–3):145–161
Roy S, Yue CY, Lam YC (2011) Influence of plasma surface treatment on thermal bonding and flow behavior in cyclic olefin copolymer (COC) based microfluidic devices. Vacuum 85(12):1102–1104
Jena RK, Yue CY, Lam YC, Tang PS, Gupta A (2012) Comparison of different molds (epoxy, polymer and silicon) for microfabrication by hot embossing technique. Sens Actuators B Chem 163(1):233–241
Leech P, Zhang X, Zhu Y (2010) Effect of norbornene content on deformation properties and hot embossing of cyclic olefin copolymers. J Mater Sci 45(19):5364–5369. doi:10.1007/s10853-010-4585-2
Tsao CW, Hromada L, Liu J, Kumar P, DeVoe DL (2007) Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 7(4):499–505
Sung D, Shin DH, Jon S (2011) Toward immunoassay chips: facile immobilization of antibodies on cyclic olefin copolymer substrates through pre-activated polymer adlayers. Biosens Bioelectron 26(9):3967–3972
Raj J, Herzog G, Manning M, Volcke C, MacCraith BD, Ballantyne S, Thompson M, Arrigan DWM (2009) Surface immobilisation of antibody on cyclic olefin copolymer for sandwich immunoassay. Biosens Bioelectron 24(8):2654–2658
Emiliyanov G, Høiby P, Pedersen L, Bang O (2013) Selective serial multi-antibody biosensing with TOPAS microstructured polymer optical fibers. Sensors 13(3):3242
Emiliyanov G, Jensen JB, Bang O, Hoiby PE, Pedersen LH, Kjær EM, Lindvold L (2007) Localized biosensing with Topas microstructured polymer optical fiber. Opt Lett 32(5):460–462
Liu K, Fan ZH (2011) Thermoplastic microfluidic devices and their applications in protein and DNA analysis. The Analyst 136(7):1288–1297
Diaz-Quijada GA, Peytavi R, Nantel A, Roy E, Bergeron MG, Dumoulin MM, Veres T (2007) Surface modification of thermoplastics: towards the plastic biochip for high throughput screening devices. Lab Chip Miniat Chem Biol 7(7):856–862
Bhattacharyya A, Klapperich CM (2007) Design and testing of a disposable microfluidic chemiluminescent immunoassay for disease biomarkers in human serum samples. Biomed Microdevices 9(2):245–251
Jönsson C, Aronsson M, Rundström G, Pettersson C, Mendel-Hartvig I, Bakker J, Martinsson E, Liedberg B, MacCraith B, Öhman O, Melin J (2008) Silane-dextran chemistry on lateral flow polymer chips for immunoassays. Lab Chip Miniat Chem Biol 8(7):1191–1197
Liu Y, Cady NC, Batt CA (2007) A plastic microchip for nucleic acid purification. Biomed Microdevices 9(5):769–776
Laib S, MacCraith BD (2007) Immobilization of biomolecules on cycloolefin polymer supports. Anal Chem 79(16):6264–6270
Petrtyl M, Bastl Z, Krulis Z, Hulejova H, Polanska M, Lisal J, Danesova J, Cerny P (2010) Cycloolefin-copolymer/polyethylene (COC/PE) blend assists with the creation of new articular cartilage. Macromol Symp 294(1):120–132
Chang S-C, Lee M-J, Lin H-M (2007) Nanoparticles formation for metallocene catalyzed cyclic olefin copolymer via a continuous supercritical anti-solvent process. J Supercrit Fluids 40(3):420–432
Pegoretti A, Dorigato A, Biani A, Slouf M (2016) Cyclic olefin copolymer–silica nanocomposites foams. J Mater Sci 51(8):3907–3916. doi:10.1007/s10853-015-9710-9.
Rwei S-P, Lin Y-T, Su Y-Y (2012) Investigation on the spinnability of metallocene cyclic olefins copolymer melt. Text Res J 82(4):315–323
Saadé J, Declas N, Marote P, Bordes C, Faure K (2017) Functionalization of cyclic olefin copolymer substrates with polyethylene glycol diacrylate for the in situ synthesis of immobilized nanoparticles. J Mater Sci 52(8):4509–4520. doi: 10.1007/s10853-016-0696-8
Brauer E, Wiegleb H, Helmstedt M (1986) Characterization of ethylene homo- and copolymers. Polym Bull 15(6):551–557
Brauer E, Wild C, Wiegleb H (1987) Characterization of ethylene homo- and copolymers. Polym Bull 18(1):73–80
Yamazaki M (2004) Industrialization and application development of cyclo-olefin polymer. J Mol Catal A Chem 213(1):81–87
Blank F, Janiak C (2009) Metal catalysts for the vinyl/addition polymerization of norbornene. Coord Chem Rev 253(7–8):827–861
Heinz BS, Alt FP, Heitz W (1998) Pd(II)-catalyzed vinylic polymerization of norbornene and copolymerization with norbornene and copolymerization with norbornene carboxylic acid esters. Macromol Rapid Commun 19(5):251–256
Li Y, Gao M, Wu Q (2007) Vinyl polymerization of norbornene by nickel(II) complexes bearing β-diketiminate ligands. Appl Organomet Chem 21(11):965–969
Suslov DS, Bykov MV, Abramov PA, Pakhomova MV, Ushakov IA, Voronov VK, Tkach VS (2016) Synthesis, characterization, and application for addition polymerization of norbornene of novel acetylacetonate bis(anilines) palladium (II) complexes. Inorg Chem Commun 66:1–4
Wang Y-Y, Lin S-A, Zhu F-M, Gao H-Y, Wu Q (2008) Dinuclear nickel (II) complexes bearing two pyrazolylimine ligands: synthesis characterization, and catalytic properties for vinyl-type polymerization of norbornene. Polym Degrad Stab 44(7):2308–2317
Kaminsky W, Spiehl R (1989) Copolymerization of cycloalkenes with ethylene in presence of chiral zirconocene catalysts. Die Makromol Chem 190(3):515–526
Kaminsky W, Bark A, Arndt M (1991) New polymers by homogenous zirconocene/aluminoxane catalysts. Makromol Chem Macromol Symp 47(1):83–93
Kaminsky W (2004) The discovery of metallocene catalysts and their present state of the art. J Polym Sci Part A Polym Chem 42(16):3911–3921
Yoshida Y, Matsui S, Fujita T (2005) Bis(pyrrolide-imine) Ti complexes with MAO: a new family of high performance catalysts for olefin polymerization. J Organomet Chem 690(20):4382–4397
Lawrence WH, Turner JE, Autian J (1969) Reevaluation of plastic tubings currently used in medical and paramedical applications. J Biomed Mater Res 3(2):291–303
Ruchatz D, Fink G (1998) Ethene norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: kinetics and relationships between catalyst structure and polymer structure. 1. Kinetics of the ethene norbornene copolymerization using the [(Isopropylidene)(η5-inden-1-ylidene-η5-cyclopentadienyl)]zirconium dichloride/methylaluminoxane catalyst. Macromolecules 31(15):4669–4673
Ruchatz D, Fink G (1998) Ethene norbornene copolymerization using homogenous metallocene and half-sandwich catalysts: kinetics and relationships between catalyst structure and polymer structure. 2. Comparative study of different metallocene- and half-sandwich/methylaluminoxane catalysts and analysis of the copolymers by 13C nuclear magnetic resonance spectroscopy. Macromolecules 31(15):4674–4680
Ruchatz D, Fink G (1998) Ethene norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: kinetics and relationships between catalyst structure and polymer structure. 3. Copolymerization parameters and copolymerization diagrams. Macromolecules 31(15):4681–4683
Wendt RA, Fink G (2003) Ethene-norbornene copolymerizations using two different homogeneous metallocene catalyst systems and investigations of the copolymer microstructure. J Mol Catal A Chem 203(1–2):101–111
Tritto I, Boggioni L, Ferro DR (2004) Alternating isotactic ethylene norbornene copolymers by C1-symmetric metallocenes: determination of the copolymerization parameters and mechanistic considerations on the basis of pentad analysis. Macromolecules 37(26):9681–9693
Yao Z, Lv F, Liu S-J, Cao K (2008) Synthesis of ethylene and norbornene copolymer with metallocene catalysts and characteristic analysis. J Appl Polym Sci 107(1):286–291
Li X, Hou Z (2008) Organometallic catalysts for copolymerization of cyclic olefins. Coord Chem Rev 252(15–17):1842–1869
Liu S, Yao Z, Cao K, Li B, Zhu S (2009) Preparation of polar ethylene–norbornene copolymers by metallocene terpolymerization with triisobutylaluminium-protected but-3-en-1-ol. Macromol Rapid Commun 30(7):548–553
Ruchatz D, Fink G (1998) Ethene norbornene copolymerization with homogeneous metallocene and half-sandwich catalysts: kinetics and relationships between catalyst structure and polymer structure. 4. Development of molecular weights. Macromolecules 31(15):4684–4686
TOPAS, Cyclic Olefin Copolymer, TOPAS ADVANCED POLYMERS, www.topas.com (last viewed May 2015)
Mitsui C Grades and properties of APEL. www.mitsuichemical.com/apel.htm (last viewed May 2015)
Blank F, Scherer H, Janiak C (2010) Oligomers and soluble polymers from the vinyl polymerization of norbornene and 5-vinyl-2-norbornene with cationic palladium catalysts. J Mol Catal A Chem 330(1–2):1–9
Floros G, Saragas N, Paraskevopoulou P, Psaroudakis N, Koinis S, Pitsikalis M, Hadjichristidis N, Mertis K (2012) Ring opening metathesis polymerization of norbornene and derivatives by the triply bonded ditungsten complex Na[W2(µ-Cl)3Cl4(THF)2]·(THF)3. Polymers 4(4):1657
Cui J, Yang J-X, Li Y-G, Li Y-S (2015) Synthesis of high performance cyclic olefin polymers (COPs) with ester group via ring-opening metathesis polymerization. Polymers 7(8):1389
ZEONEX C Cyclo olefin polymer (COP) ZEONEX. www.zeon.co.jp/content (last viewed May 2015)
COP Z Cyclo olefin polymer (COP) ZEONOR. www.zeon.co.jp/content (last viewed May 2015)
Isasi JR, Haigh JA, Graham JT, Mandelkern L, Alamo RG (2000) Some aspects of the crystallization of ethylene copolymers. Polymer 41(25):8813–8823
Shin JUY, Park JIY, Liu C, He J, Kim SC (2005) Chemical structure and physical properties of cyclic olefin copolymers (IUPAC technical report). Pure Appl Chem 77(5):801–814
Seydewitz V, Krumova M, Michler GH, Park JY, Kim SC (2005) Morphology and micromechanical behaviour of ethylene cycloolefin copolymers (COC). Polymer 46(15):5608–5614
Rische T, Waddon AJ, Dickinson LC, MacKnight WJ (1998) Microstructure and morphology of cycloolefin copolymers. Macromolecules 31(6):1871–1874
Thorshaug K, Mendichi R, Boggioni L, Tritto I, Trinkle S, Friedrich C, Malhaupt R (2002) Poly(ethene-co-norbornene) obtained with a constrained geometry catalyst. A study of reaction kinetics and copolymer properties. Macromolecules 35(8):2903–2911
Li Y, Gao M, Gao H, Wu Q (2011) Synthesis and structural characterization of ethylene–norbornene copolymer with high norbornene content catalyzed by β-diketiminato nickel/methylaluminoxane. Polym Degrad Stab 47(10):1964–1969
Bergström C, Ruotoistenmäki J, Seppälä J (1997) A GPC method to determine the amount of comonomer in an ethylene–norbornene-copolymer. Polym Test 16(1):43–48
Chu PPJ, Huang WJ, Chang FC (2001) Kinetics for the growth of rigid amorphous fraction in cyclic olefin copolymers (COC). Polymer 42(5):2185–2191
Tritto I, Boggioni L, Sacchi MC, Locatelli P, Ferro DR, Provasoli A (1999) Ethylene–norbornene copolymers prepared with metallocene-based catalysts: new sequence assignments by 13C NMR. Macromol Rapid Commun 20(5):279–283
Arndt-Rosenau M, Beulich I (1999) Microstructure of ethene/norbornene copolymers. Macromolecules 32(22):7335–7343
Tritto I, Marestin C, Boggioni L, Sacchi MC, Brintzinger H-H, Ferro DR (2001) Stereoregular and stereoirregular alternating ethylene–norbornene copolymers. Macromolecules 34(17):5770–5777
Benavente R, Scrivani T, Cerrada ML, Zamfirova G, Pérez E, Pereña JM (2003) Glass-transition temperature determination by microhardness in norbornene–ethylene copolymers. J Appl Polym Sci 89(13):3666–3671
Forsyth J, Pereña JM, Benavente R, Pérez E, Tritto I, Boggioni L, Brintzinger H-H (2001) Influence of the polymer microstructure on the thermal properties of cycloolefin copolymers with high norbornene contents. Macromol Chem Phys 202(5):614–620
Arndt M, Beulich I (1998) C1-symmetric metallocenes for olefin polymerisation, 1. Catalytic performance of [Me2C(3-tertBuCp)(Flu)]ZrCl2 in ethene/norbornene copolymerisation. Macromol Chem Phys 199(6):1221–1232
Park SY, Choi KY, Song KH, Jeong BG (2003) Kinetic modeling of ethylene–norbornene copolymerization using homogeneous metallocene catalysts. Macromolecules 36:4216–4225
Kaminsky W, Tran P-D, Werner R (2004) New polymers by copolymerization of ethylene and norbornene with metallocene catalysts. Macromol Symp 213(1):101–108
Sacchi MC, Sonzogni M, Losio S, Forlini F, Locatelli P, Tritto I, Licchelli M (2001) Vinylic polymerization of norbornene by late transition metal-based catalysis. Macromol Chem Phys 202(10):2052–2058
Wilson TP, Von Dohlen WC, Koleske JV (1974) Copolymers of ethylene with norbornene derivatives—dynamic mechanical properties. J Polym Sci Polym Phys Ed 12(8):1607–1618
Forsyth JF, Scrivani T, Benavente R, Marestin C, Pereña JM (2001) Thermal and dynamic mechanical behavior of ethylene/norbornene copolymers with medium norbornene contents. J Appl Polym Sci 82(9):2159–2165
Scrivani T, Benavente R, Pérez E, Pereña JM (2001) Stress–strain behaviour, microhardness, and dynamic mechanical properties of a series of ethylene–norbornene copolymers. Macromol Chem Phys 202(12):2547–2553
Makrocka-Rydzyk M, Nowaczyk G, Glowinkowski S, Jurga S (2010) Dynamic mechanical study of molecular dynamics in ethylene–norbornene copolymers. Polymer 51(4):908–912
Makrocka-Rydzyk M, Orozbaev B, Nowaczyk G, Glowinkowski S, Jurga S (2005) Molecular dynamics in cyclic olefin copolymer. Acta Phys Pol A 108(2):385–393
Harrington BA, Crowther DJ (1998) Stereoregular, alternating ethylene–norbornene copolymers from monocyclopentadienyl catalysts activated with non-coordinating discrete anions. J Mol Catal A Chem 128(1–3):79–84
Liu C, Yu J, Sun X, Zhang J, He J (2003) Thermal degradation studies of cyclic olefin copolymers. Polym Degrad Stab 81(2):197–205
Ekizoglou N, Thorshaug K, Cerrada ML, Benavente R, Pérez E, Pereña JM (2003) Influence of the molecular weight on the thermal and mechanical properties of ethylene/norbornene copolymers. J Appl Polym Sci 89(12):3358–3363
Dorigato A, Pegoretti A, Fambri L, Lonardi C, Slouf M, Kolarik J (2011) Linear low density polyethylene/cycloolefin copolymer blends. Express Polym Lett 5(1):23–37
Liu MO, Lin H-F, Yang M-C, Lai M-J, Chang C-C, Feng M-C, Shiao P-L, Chen I-M (2006) Thermal oxidation and molding feasibility of cycloolefin copolymers (COCs) with high glass transition temperature. Polym Degrad Stab 91(7):1443–1447
Liu MO, Lin H-F, Yang M-C, Lai M-J, Chang C-C, Shiao P-L, Chen I-M, Chen J-Y (2007) Thermal, dynamic mechanical and rheological properties of metallocene-catalyzed cycloolefin copolymers (mCOCs) with high glass transition temperature. Mater Lett 61(2):457–462
Saunier J, Aymes-Chodur C, Leclerc B, Larbes L, Yagoubi N (2008) Using COC as pharmaceutical or cosmetic storage material: part II. Effect of electron beam radio-sterilisation. J Appl Polym Sci 109:1829–1839
Barakat H, Aymes-Chodur C, Saunier J, Yagoubi N (2013) Effect of electron beam radio sterilization on cyclic olefin copolymers used as pharmaceutical storage materials. Radiat Phys Chem 84:223–231
Delpech MC, Coutinho FMB, Habibe MES (2002) Viscometry study of ethylene–cyclic olefin copolymers. Polym Test 21(4):411–415
Saunier J, Aymes-Chodur C, Larbes L, Mrad O, Yagoubi N (2007) Using cycloolefin copolymers as pharmaceutical or cosmetic storage material. I. A study of adjuvant migration and of polymer ageing. J Appl Polym Sci 104(1):585–593
Šećerov B, Cincović MTM, Popović S, Nedić Z, Kaćarević-popović Z (2008) Characterization of gamma irradiated ethylene–norbornene copolymer using FTIR, UV–Vis and DSC techniques. Polym Bull 60(2):313–322
Paskiet D, Jenke D, Ball D, Houston C, Norwood DL, Markovic I (2013) The Product Quality Research Institute (PQRI) leachables and extractables working group initiatives for parenteral and ophthalmic drug product (PODP). PDA J Pharm Sci Technol 67(5):430–447
Pilař J, Michálková D, Šeděnková I, Pfleger J, Pospíšil J (2011) NOR and nitroxide-based HAS in accelerated photooxidation of carbon-chain polymers; comparison with secondary HAS: an ESRI and ATR FTIR study. Polym Degrad Stab 96(5):847–862
White JR, Turnbull A (1994) Weathering of polymers: mechanisms of degradation and stabilization, testing strategies and modelling. J Mater Sci 29(3):584–613. doi: 10.1007/BF00445969
Aymes-Chodur C, Sghaïer M, Yagoubi N (2016) Thermo-oxidative stability of electron beam irradiated ethylene norbornene copolymer. Radiat Phys Chem 118:128–132
Gutiérrez-Villarreal MH, Zavala-Betancourt SA (2014) Thermo-oxidative stability of cyclic olefin copolymers in the presence of Fe, Co and Mn stearates as pro-degradant additives. Polym Plast Technol Eng 53(17):1804–1810
Yang TCK, Lin SSY, Chuang T-H (2002) Kinetic analysis of the thermal oxidation of metallocene cyclic olefin copolymer (mCOC)/TiO2 composites by FTIR microscopy and thermogravimetry (TG). Polym Degrad Stab 78(3):525–532
Jakubowicz I, Enebro J (2012) Effects of reprocessing of oxobiodegradable and non-degradable polyethylene on the durability of recycled materials. Polym Degrad Stab 97(3):316–321
Klein JM, Ramos GR, Grisa AMC, Coulon GR, Brandalise RN, Zeni M (2013) Thermogravimetric and morphologic analysis of oxo-degradable polyethylene films after accelerated weathering. Progr Rubber Plast Recycl Technol 29(1):39–54
Grassie N, Farish ME (1967) The photo degradation of copolymers of methyl methacrylate and acrylonitrile. Eur Polym J 3(4):627–635
Nakade K, Nagai Y, Ohishi F (2010) Photodegradation of some ethylene–norbornene random copolymers. Polym Degrad Stab 95(12):2654–2658
Pilař J, Michálková D, Šlouf M, Vacková T, Dybal J (2014) Heterogeneity of accelerated photooxidation in commodity polymers stabilized by HAS: ESRI, IR, and MH study. Polym Degrad Stab 103:11–25
Jena RK, Yue CY, Anand L (2011) Improvement of thermal bond strength and surface properties of cyclic olefin copolymer (COC) based microfluidic device using the photo-grafting technique. Sens Actuators B Chem 157(2):518–526
Jena RK, Yue CY (2012) Cyclic olefin copolymer based microfluidic devices for biochip applications: ultraviolet surface grafting using 2-methacryloyloxyethyl phosphorylcholine. Biomicrofluidics 6(1):012822-1–012822-12
Barakat H, Saunier J, Aymes-Chodur C, Aubert P, Vigneron J, Etcheberry A, Yagoubi N (2013) Modification of a cyclo-olefin surface by radio-sterilization: is there any effect on the interaction with drug solutions. Int J Pharm 456(1):212–222
Cerrada ML, Hermida I, Benavente R, Ressia J, Vallés EM (2011) Branching and rheological behavior after electron irradiation in metallocene ethylene-co-norbornene copolymers. Polym Test 30(1):35–42
Hwang S-J, Tseng M-C, Shu J-R, Her Yu H (2008) Surface modification of cyclic olefin copolymer substrate by oxygen plasma treatment. Surf Coat Technol 202(15):3669–3674
Kačarević-Popović Z, Šećerov B, Marinović-Cincović MT, Nedić Z, Slobodan MJ (2006) Modification of ethylene–norbornene copolymer by Gamma irradiation. Hem Ind Vol Issue Pages 60(11–12):311–315
Kačarević-Popović Z, Tjapkin N, Siljegović M, Draganić I (2005) Surface modification of ethylene–norbornene copolymer by irradiation with N4+ ion beams. NIM B 236(1–4):594–598
Haji-Saeid M, Sampa MHO, Chmielewski AG (2007) Radiation treatment for sterilization of packaging materials. Radiat Phys Chem 76(8–9):1535–1541
Šiljegović M, Kačarević-Popović ZM, Krklješ AN, Stojanović Z, Jovanović ZM (2011) Effect of N4+ and C4+ ion beam bombardment on the optical and structural characteristics of ethylene–norbornene copolymer (TOPAS). NIM B 269(7):708–715
Chapiro A (1988) Chemical modifications in irradiated polymers. NIM B32:111–114
Clough RL (2001) High-energy radiation and polymers: a review of process and emerging applications. Nucl Instrum Methods Phys Res B 185:8–33
Cambon S (2001) Etude du mécanisme de dégradation radiochimique d’un élastomère de type EPDM, Sciences fondamentales. Blaise Pascal, Clermont Ferrand
Clark DT, Dilks A (1978) ESCA applied to polymers. XVIII. RF glow discharge modification of polymers in helium, neon, argon, and krypton. J Polym Sci Polym Chem Ed 16(5):911–936
Johansson B-L, Larsson A, Ocklind A, Öhrlund Å (2002) Characterization of air plasma-treated polymer surfaces by ESCA and contact angle measurements for optimization of surface stability and cell growth. J Appl Polym Sci 86(10):2618–2625
Nikolova D, Dayss E, Leps G, Wutzler A (2004) Surface modification of cycloolefinic copolymers for optimization of the adhesion to metals. Surf Interface Anal 36(8):689–693
Polanska M, Hulejova H, Petrtyl M, Bastl Z, Spirovova I, Krulis Z, Horak Z, Veigl D, Senolt L (2010) Surface modification of cyclic olefin-copolymers for osteochondral defect repair can increase pro-destructive potential of human chondrocytes in vitro. Physiol Res 59:247–253
Kuzuya M, Niwa J, Ito H (1993) Nature of plasma-induced surface radicals of powdered polyethylene studied by electron spin resonance. Macromolecules 26(8):1990–1995
Kuzuya M, Kondo SI, Sugito M, Yamashiro T (1998) Peroxy radical formation from plasma-induced surface radicals of polyethylene as studied by electron spin resonance. Macromolecules 31(10):3230–3234
Mix R, Friedrich JF, Neubert D, Inagaki N (2014) Response of linear, branched or crosslinked polyethylene structures on the attack of oxygen plasma. Plasma Chem Plasma Process 34(5):1199–1218
Kettner P, Pelzer RL, Glinsner T, Farrens S (2006) New results on plasma activated bonding of imprinted polymer features for bio MEMS applications. J Phys Conf Ser 34:65–71
Fintzou AT, Badeka AV, Kontominas MG, Riganakos KA (2006) Changes in physicochemical and mechanical properties of [gamma]-irradiated polypropylene syringes as a function of irradiation dose. Radiat Phys Chem 75(1):87–97
AFNOR-NF-EN-552, AFNOR NF (1994) EN. 552 Stérilisation de dispositifs médicaux. Validation et contrôle de routine pour la stérilisation par irradiation
Šećerov B, Kačarević-Popović ZM, Marinović-Cincović MT, Nedić Z, Jovanović SM (2006) Modification of ethylene–norbornene copolymer by Gamma irradiation. Hem Ind 60:311–315
Ansari IA, Datta AK (2003) An overview of sterilization methods for packaging materials used in aseptic packaging systems. Food Bioprod Process 81(1):57–65
Fintzou AT, Kontominas MG, Badeka AV, Stahl MR, Riganakos KA (2007) Effect of electron-beam and gamma-irradiation on physicochemical and mechanical properties of polypropylene syringes as a function of irradiation dose: study under vacuum. Radiat Phys Chem 76(7):1147–1155
Davenas J, Stevenson I, Celette N, Cambon S, Gardette JL, Rivaton A, Vignoud L (2002) Stability of polymers under ionising radiation: the many faces of radiation interactions with polymers. NIM B 191(1–4):653–661
Majumder AKBPS (1999) Surface and bulk-properties of EPDM rubber modified by electron beam irradiation. Radiat Phys Chem 53:63–78
Novakovic L, Gal O (1995) Irradiation effect on polyethylene in the presence of an antioxidant and trifunctional monomers. Polym Degrad Stab 50(1):53–58
Gal O, Novaković L, Marković V, Stannett VT (1977) Thermogravimetric studies of the thermooxidative stability of irradiated and unirradiated polyethylene part I. Effect of antioxidant. Radiat Phys Chem 22(3–5):627–634
Zaharescu T, Giurginca M, Jipa S (1999) Radiochemical oxidation of ethylene–propylene elastomers in the presence of some phenolic antioxidants. Polym Degrad Stab 63(2):245–251
Sharma T, Aggarwal S, Sharma A, Kumar S, Kanjilal D, Deshpande SK, Goyal PS (2007) Effect of nitrogen ion implantation on the optical and structural characteristics of CR-39 polymer. J Appl Phys 102(6):063527
Shah S, Qureshi A, Singh NL, Kulriya PK, Singh KP, Avasthi DK (2009) Structural and chemical modification of polymer composite by proton irradiation. Surf Coat Technol 203(17–18):2595–2599
Švorčík V, Prošková K, Hnatowicz V, Arenholz E, Kluge A (1999) Polyimide modified by irradiation with C+ and N+ ion beams. Polym Degrad Stab 65(1):131–135
Allen DW, Clench MR, Crowson A, Leathard DA, Saklatvala R (1994) Characterisation of electron beam generated transformation products of irganox 1010 by particle beam liquid chromatography-mass spectrometry with on-line diode array detection. J Chromatogr A 679(2):285–297
El Mansouri H, Yagoubi N, Ferrier D (1998) Extraction of polypropylene additives and their analysis by HPLC. Chromatographia 48(7/8):491–496
Aymes-Chodur C, Betz N, Legendre B, Yagoubi N (2006) Structural and physico-chemical studies on modification of polypropylene and its polyphenolic antioxidant by electron beam irradiation. Polym Degrad Stab 91(4):649–662
Burman L, Albertsson A-C, Höglund A (2005) Solid-phase microextraction for qualitative and quantitative determination of migrated degradation products of antioxidants in an organic aqueous solution. J Chromatogr A 1080(2):107–116
Bourges F, Bureau G, Pascat B (1993) Effects of electron beam irradiation on the migration of antioxidants and their degradation products from commercial polypropylene into food simulating liquids. Food Addit Contam 10(4):443–452
Lau O-W, Wong S-K (2000) Contamination in food from packaging material. J Chromatogr A 882:255–270
Jenke DR (2007) Evaluation of the chemical compatibility of plastic contact materials and pharmaceutical products; safety considerations related to extractables and leachables. J Pharm Sci 96(10):2566–2581
Allen DW, Leathard DA, Smith C (1991) The effects of gamma irradiation on the fate of hindered phenol antioxidants in food contact polymers. Analytical and 14C-labelling studies. Int J Radiat Appl Instrum Part C Radiat Phys Chem 38(5):461–465
Allen DW, Clench MR, Crowson A, Leathard DA (1993) Identification by particle-beam liquid chromatography-mass spectrometry of transformation products of the antioxidant Irganox 1330 in food-contact polymers subjected to electron-beam irradiation. J Chromatogr A 629(2):283–290
Allen DW, Clench MR, Crowson A, Leathard DA (1993) Characterisation of solvent-extractable transformation products of high molecular weight hindered phenols in polypropylene subjected to ionising radiation in air or to thermal ageing. Polym Degrad Stab 39:293–297
Juo CG, Chen SW, Her GR (1995) Mass spectrometric analysis of additives in polymer extracts by desorption chemical ionization and collisional induced dissociation with B/E linked scanning. Anal Chim Acta 311(2):153–164
Jackson AT, Jennings KR, Scrivens JH (1996) Analysis of a five-component mixture of polymer additives by means of high energy mass spectrometry and tandem mass spectrometry. Rapid Commun Mass Spectrom 10(12):1449–1458
Block C, Wynants L, Kelchtermans M, De Boer R, Compernolle F (2006) Identification of polymer additives by liquid chromatography-mass spectrometry. Polym Degrad Stab 91(12):3163–3173
Fries Elke W (2002) Puttmann, analysis of the antioxidant butylated hydroxytoluene (BHT) in water by means of solid phase extraction combined with GC/MS. Water Res 36:2319–2327
Jenke D (2006) Extractable substances from plastic materials used in solution contact applications: an updated review. PDA J Pharm Sci Technol 60(3):191–207
Jenke D, Castner J, Egert T, Feinberg T, Hendricker A, Houston C, Hunt DG, Lynch M, Shaw A, Nicholas K, Norwood DL, Paskiet D, Ruberto M, Smith EJ, Holcomb F (2013) Extractables characterization for five materials of construction representative of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Technol 67(5):448–511
P. Biotechnology, Pierce_LDH_Cytotoxicity_Assay_Kit, Thermo scientific (last viewed May 2015)
Tim M (1983) Rapidcolorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Grabowski N, Hillaireau H, Vergnaud J, Tsapis N, Pallardy M, Kerdine-Romer S, Fattal E (2015) Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int J Pharm 482(1–2):75–83
Egesten AS, Herwald A (2008) Trends in innate immunity. Contrib Microbiol 15:164–187
van Midwoud PM, Janse A, Merema MT, Groothuis GM, Verpoorte E (2012) Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal Chem 84(9):3938–3944
Acknowledgements
Authors would like to thank Ticona Company (Germany) for kindly providing the ENC samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lago, W.S.R., Aymes-Chodur, C., Ahoussou, A.P. et al. Physico-chemical ageing of ethylene–norbornene copolymers: a review. J Mater Sci 52, 6879–6904 (2017). https://doi.org/10.1007/s10853-017-0925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0925-9