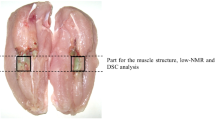
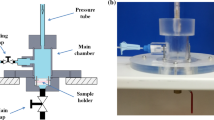
Abstract
Increasingly evidence seems that from the biological samples—mainly the summation of DSC scans of the human blood plasma—can be separated into main and well-known components by deconvolution method. This fact alone could be an important advance in thermoanalytical research, but mostly, it has not been detected in soft tissues yet. The other main reason was that in our previous studies, the histological examinations did not show any significant abnormalities in the intestinal wall muscle layer. Thus, the aim of current research was to measure the small intestinal muscular tissue scans by deconvolution method following different long warm and cold ischaemia animal experiments. Retrospectively, DSC curves obtained from the thermoanalysis of intestinal tissue in animal experiments investigated by deconvolution mathematical methods. Different warm ischaemic insults caused mild decrease after 3 and 6 h in Tm3 and Tm4 transitions in the myosin assigned transition and actin filament transition. After cold storage, the separated 5 melting components appeared in similar order, but the decreases were lesser than after warm ischaemia. Meanwhile, the calorimetric enthalpy which is a good monitor of intervention is decreased in a time-dependent manner after 1 and 3 h in warm and cold ischaemia cases. In contrast, the enthalpy increased above the control value after 6-h warm or cold tissue damage. These results confirmed that both warm and cold ischaemic injuries are detectable by deconvolution of DSC curves in the muscular intestinal layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small intestine is one of the most susceptible organs to ischaemia/reperfusion (I/R) injury. According to the origin and clinicopathological processes of intestinal I/R conditions should be caused by warm ischaemia (blood stasis inside mesenteric vessels at body temperature) or by cold ischaemia (organ storage in preservation solution among melting ice).
Based on data from major clinical centres, it is now common knowledge, that warm intestinal ischaemia, namely acute mesenteric ischaemia (AMI), leads to death in 60–90% of patients. AMI is a potentially catastrophic abdominal condition with a prevalence of 1‰ (i.e. 1 person in 1000 acute hospital admissions) [1]. Various long-term cold ischaemia periods (storage) are necessary and inevitable during small bowel allograft transplantation. Today, bowel transplantation is only the permanent and curative treatment for intestinal failure. For any organ, it is true that, despite the drawbacks or complications of transplantation, the life expectancy, survival, and patients’ life quality can be considered far superior to the therapeutic outcomes of "artificial organs" [2, 3].
Rich vascular system of the intestinal tissue, its extensive collateral and natural anastomosis system (arc of Barkow, arc of Bühler, arc of Riolan, and Drummond's arcades, intramural submucosal vascular plexuses), and its extensive compensatory mechanisms (e.g. intramural redistribution, regulation of the efficiency of oxygen extraction, dynamic alternation of vasoconstriction and vasodilation) provide multiple levels of protection. But, the structure and neural-hormonal regulation of the mesenteric circulation also explain why the gut, and within it its mucosa is one of the most sensitive tissues to ischaemic effects. In focal non-transmural lesions, mucosal necrosis, submucosal oedema, haemorrhages, and mucosal ulcerations are observed, which can heal spontaneously with fibrotic scarring, possibly leaving a stenosis. Damage extending over the entire intestinal wall usually develops due to acute and fatal ischaemic effects causing transmural necrosis, gangrene, and perforation [4].
Routinely, detection of intestinal mucosa damage is based on histological examination of haematoxylin and eosin-stained sections. But there is no significant change in the muscle layer of the intestinal wall with this method. Meanwhile, it is an empirical fact in surgical operations that after intestinal operations, the function and peristalsis of the intestine are paralysed and damaged for a longer and shorter period [5]. In several previous studies, our experimental group demonstrated that warm and cold I/R injury of the small intestine is detectable not only with light microscope but with calorimeter device as well [6,7,8]. Over the past decades, great advances have been made in many areas of the biological sample experiments, including involving differential scanning calorimetry (DSC) measurements to the medicine. Blood plasma analysis of oncological patients has come to the forefront [9,10,11,12]. Another forward step in research, when the use of deconvolution methods has become increasingly common with the further analysis of DSC curves [13,14,15]. A recent advance was the study in which the blood plasma proteome of colorectal cancer patients was classified, deconvoluted and subdivided based on the derived thermodynamic parameters. And by doing so, they have shown and confirmed proof of principle that the DSC technique is suitable for monitoring changes in the blood plasma proteome of patients with cancer [16].
Overlapping thermal transitions observed in DSC experiments can be resolved to varying levels of success using numerical deconvolution methods. Even before biological application, the components of the summed curves were detected and resolved such as commonly known in thermal data analysis process also [17, 18]. These facts encouraged us to use deconvolution method not only in a liquid sample such as a blood plasma, but in a muscular layer of structured soft tissue such as the main functional part of small intestinal wall.
Materials and methods
Experimental protocol
Animal experiments have been published previously [7, 8]. Briefly, on adult, male, general anaesthetized Wistar rats (250–300 g) warm ischaemia groups on body temperature were established after median laparotomy with the occlusion of superior mesenteric artery (SMA) for 1, 3 and 6 h (n = 5/each group). In cold ischaemia groups, after small bowel resection from the ligament of Treitz to the ileocecal part, the grafts were perfused and stored in 4 °C University of Wisconsin (UW) solution (Viaspan, Bristol-Myers Squibb GesmbH) for 1, 3 and 6 h. Total intestinal wall biopsies were collected after laparotomy (control) and at the end of the ischemic periods. All experiment approved by the University of Pécs (BA02/2000-20/2006, BA02/2000-9/2008).
DSC measurement
The thermal unfolding of the small bowel muscle layer was monitored by SETARAM Micro DSC-II calorimeter as previously described [7, 8]. From thermal parameters, the melting points (Tms), the calorimetric enthalpy (ΔH) and enthalpy contribution (ΔH in %) of deconvoluted curves of total intestinal wall samples have been measured both in the control tissue and following different warm or cold ischaemic periods.
Deconvolution of DSC thermal curves
As described in our previous blood plasma measurements [13,14,15], the obtained DSC scans can be decomposed into a sum of Gaussian curves that way that their total area is nearly the same as of the experimental curve one, within a reasonable error (R2 changed between 0.9507 and 0.9918). To have the best fitting we applied more than five curves, but some contribution was less than the error of enthalpy determination, so they cannot influence our final interpretation of data, we have neglected them.
Statistical analysis
All results are given in mean values ± standard error of the mean (SEM). Data were analysed with one-way ANOVA. The level of significance was set at p < 0.05.
Results
The control samples of the intestinal muscle layer showed an endotherm process with two main melting temperature (Tms) at 58.2 ± 0.2 °C and at 74.0 ± 0.2 °C with a total calorimetric enthalpy 0.46 ± 0.03 J g-1 (Table 1; Figs. 1–3A). Deconvolution of this curve resulted in 5 thermal transitions with Tm1-44.7 °C, Tm2-53.5 °C, Tm3-58.2 °C, Tm4-62.6 °C and Tm5-74.4 °C. The order of appearance each component in case of 5 transition temperature was orange, light green, navy blue, magenta, and dark yellow. From these, the Tm3 and Tm5 transitions gave the main enthalpy contribution in % (Table 1).
Warm ischaemia caused intestinal muscle layer injury that appeared already after 1-h occlusion, namely transition temperatures the Tm1, Tm3 and Tm4 increased, while Tm2 transition decreased significantly. The order of appearance of each component in case of 5 transition temperature was light green, royal blue, cyan blue, magenta, and dark yellow (and only in one case the 6th curve was signed with navy blue colour). Basically, the Tm5 was at 74 °C and did not change following 1-h warm ischaemia compared to control. The calorimetric enthalpy was 1.1 ± 0.07, which is mainly divided between Tm3 (31.8%) and Tm5 (42.5%) transitions (Table 1; Fig. 1B).
Three-hours ischaemia caused such structural changes that could be decomposed in 6 melting temperatures, where the last one was a newly appeared transition temperature at 80 °C. However, the enthalpy was 0.28 ± 0.02 J g-1, and the Tm4 with 60% was the main responsible for the enthalpy contribution (Table 1, Fig. 2B).
After 6-h warm occlusion caused the same decreased tendency in the deconvoluted Tm2 and Tm3, while Tm4 melting peak disappeared, and Tm5 increased significantly by the end of the research period. The measured calorimetric enthalpy was 0.58 ± 0.03 J g-1, to which contributed Tm3 and Tm5 transitions in nearly half and half ratio. (The other contributions were near to the enthalpy resolution limit, see Table 1; Fig. 3B.)
After 1-h cold storage, the measured main denaturation curve (black line) could be deconvoluted likewise into 5 thermal domains (coloured lines) (Table 1; Fig. 1C). Calorimetric enthalpy, as an indicator of the overall thermal stability of the whole system, showed a marked decrease after 1-h of ischemic insult, with the exception of both 6-h ischemic samples (warm and cold), where a significant increase was measured. (The cold 3-h treatment exhibited a mild decrease compared to the control, Table 1, Fig. 3C.) The deconvoluted thermal curves in 1-h cold ischaemic cases resulted in a significant decrease in thermal transition temperatures in case of Tm2–Tm3 and Tm4, compared to the control, while in case of 6-h treatment only Tm2 and Tm4 decreased significantly. Moreover, the main component of the calorimetric enthalpy contribution in all cold ischaemic cases was Tm3 (1-h: 49.02%, 3-h: 39.16%, 6-h: 61.91%).
Discussion
Over the past 10 years, research has focussed on the thermoanalytical analysis of disease-related samples. In fact, more and more publications mention the place of DSC as an analytical method in diagnostics or disease monitoring. In addition to well-established diagnostic methods, there is a need to test new assays, such as thermoanalysis of human blood plasma from patients with cancer and more recently, the deconvolution of complex DSC curves [16, 18,19,20,21]. Deconvolution is a practical method to determine a more accurate relative contribution of resolved thermal analysis events, that’s why is often used to resolve thermal data. The mathematical model used for deconvolution in our experiment shows good correlation and standard error [22].
This study investigated the changes of DSC curves after deconvolution in muscle layer of total intestinal wall after warm and cold ischaemic insult. These experiments can be considered as novel because no other results describe a layered soft tissue like small bowel such changes with deconvolution of DSC data. Having regard to the lack of comparable articles, we assume that these data showed 5 to 6 main temperature transitions not only in control muscle layer of intestinal wall, but after warm and cold ischaemia. According to our previous experiments performed on rabbit psoas muscle fibres, the denaturation temperatures could be addressed to the next muscle protein compounds: Tm1 and Tm2 myosin head, Tm3 myosin rod, Tm4 and Tm5 characterise the actin filament [23,24,25,26,27,28].
This way we can assume that the decrease in transition temperature caused by warm ischaemia (1, 3 and 6 h) represents the myosin head in muscle layer by Tm1 and Tm2. Later it can be the sign that this intervention affects the enzyme function of myosin head, because significantly altered compared with the control. Tm3 describes the thermal behaviour of myosin rod, which in skeletal myosin is very conservative, but here seems to be intervention sensitive. Among the denaturation peaks actin contribution characterising, Tm4 is more affected by the treatment. The calorimetric enthalpy is also a good monitor of intervention. This parameter is decreased in a time-dependent manner after 1 and 3 h in warm ischaemia cases. Surprisingly, the enthalpy increased above the control value during the longest-lasting, 6-h tissue damage.
In case of cold preservation, the change of calorimetric enthalpy follows the strength of treatment and differs from the control. The myosin head seems to be more effected than in case of warm ischaemia. The rod reflects only in 1-h preservation. Similarly, to the warm ischaemia, Tm5 is relatively intervention independent. In the cold tissue samples, the calorimetric enthalpy changes in groups 1 and 3 h showed the same trend as measured in the warm groups. Of these, the most interesting was the increase in enthalpy in the 6-h group with the longest duration of damage.
Basically, the outer, serous intestinal layer represented the near unchanged Tm5 at ~ 74 °C in all groups, which value and temperature stability showed a closed correlation to other researcher’s result [29].
Conclusions
In summary, warm intestinal ischaemic insult caused the changes in all muscle layer protein components in a timedependent manner as shown by the deconvoluted DSC curves. Namely, from the muscle proteins, the decrease of Tm1 and Tm2 transition temperatures represented changes in the myosin head, the intervention affected the enzyme function of myosin head, the Tm3 described the thermal behaviour of myosin rod, and among the denaturation peaks characterising actin contribution in Tm4. In addition, these changes in the muscle components were attenuated by the same duration of cold tissue storage in UW preservation solution in the small bowel. Overall, after previous physical separation of the intestinal wall structure to its muscle layer component, in this study, we can first detect with the thermal transitions and identify this layer after the deconvolution of the summarised DSC curves by mathematical method.
Data availability
There are no additional available data to upload.
References
Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZSJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the world society of emergency surgery. World J Emerg Surg. 2017;12:38–49.
Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210–9.
Vanholder R, Domínguez-Gil B, Busic M, Cortez-Pinto H, Craig JC, Jager KJ, Mahillo B, Stel VS, Valentin MO, Zoccali C, Oniscu GC. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17:554–68.
Park PO, Haglung U, Bulkley GB, Falt K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574–80.
Weledji EP. Perspectives on paralytic ileus. Acute Med Surg. 2020;7:e573.
Ferencz A, Nedvig K, Lőrinczy D. DSC examination of the intestinal tissue following ischemic injuries. Book: thermal analysis in medical application, Edited by Dénes Lőrinczy, Press: Akadémiai Kiadó, Budapest, 2011, 255–69.
Nedvig K, Ferencz A, Rőth E, Lőrinczy D. DSC examination of intestinal tissue following warm ischemia and reperfusion injury. J Therm Anal Calorim. 2009;95:775–9.
Ferencz A, Nedvig K, Lőrinczy D. DSC examination of intestinal tissue following cold preservation. Thermochim Acta. 2010;497:41–5.
Ferencz A, Fekecs T, Lőrinczy D. Differential scanning calorimetry as a new method to monitor human plasma in melanoma patients with regional limph node or distal metastases. In: Yaguang X, editor. Skin cancer book. InTech; 2011.
Moezzi M, Ferencz A, Lőrinczy D. Evaluation of blood plasma changes by differential scanning calorimetry in psoriatic patients treated with drugs. J Therm Anal Calorim. 2014;116:557–62.
Ferencz A, Lőrinczy D. DSC measurements of blood plasma on patients with chronic pancreatitis, operable and inoperable pancreatic adenocarcinoma. J Therm Anal Calorim. 2017;127:1187–92.
Ferencz A, Nedvig K, László E, Magyarlaki T, Lőrinczy D. DSC examination of kidney tissue following warm ischemia and reperfusion injury. Thermochim Acta. 2011;525:161–6.
Lőrinczy D, Moezzi M, Ferencz A. Deconvoluted plasma DSC curves on patients with psoriasis. J Therm Anal Calorim. 2020;142:789–96.
Lőrinczy D, Ferencz A. Comparison of deconvoluted plasma DSC curves on patients with solid tumors. J Therm Anal Calorim. 2020;142:1243–8.
Ferencz A, Lőrinczy D. Surgical stress detection in human blood plasma by DSC. J Therm Anal Calorim. 2020;142:783–8.
Todinova S, Krumova S, Kurtev P, Dimitrov V, Djongov L, Dudunkov Z, Taneva SG. Calorimetry-based profiling of blood plasma from colorectal cancer patients. Biochim Biophys Acta -General Sub. 2012;1820:1879–85.
Michael A, Zhou Z, Yavuz M, Khan M. Deconvolution of overlapping peaks from differential scanning calorimetry analysis for multi-phase Niti alloys. Thermochim Acta. 2018;665:53–9.
Vega S, Garcia-Gonzalez MA, Lanas A, Velazquez-Campoy A, Abian O. Deconvolution analysis for classifying gastric adenocarcinoma patients based on differential scanning calorimetry serum thermograms. Sci Rep. 2015;5:1–8.
Krumova S, Balansky R, Danailova A, Ganchev G, Djongov L, Gartcheva L, Taneva SG, Todinova S. Calorimetric assay to follow colorectal cancer development in experimental rat models. Thermochim Acta. 2020;691:178723.
Garbett NC, Mekmaysy CS, DeLeeuw L, Chaires JB. Clinical application of plasma thermograms. utility, practical approaches and considerations. Methods. 2015;76:41–50.
Michnik A, Drzazga Z. Thermal denaturation of mixtures of human serum proteins. DSC study J Therm Anal Calorim. 2010;101:513–8.
Nobuyoshi K. Thermoanalytical methods: fundamental principles and features. In: Pielichowski K, Pielichowska K, editors. Thermal analysis of polymeric materials: methods and developments. Wiley; 2022.
Dergez T, Könczöl F, Farkas N, Belagyi J, Lőrinczy D. DSC study of glycerol-extracted muscle fibers in intermediate states of ATP hydrolysis. J Thermal Anal Calorim. 2005;80:445–9.
Lőrinczy D, Belágyi J. Intermediate states of myosin head during ATP hydrolysis cycle in psoas muscle fibres by EPR and DSC. J Therm Anal Calorim. 2007;90:611–21.
Dergez T, Lőrinczy D, Könczöl F, Belagyi FN. Differential scanning calorimetry study of glycerinated rabbit psoas muscle fibres in intermediate state of ATP hydrolysis. BMC Struct Biol. 2007;7:41–50.
Lőrinczy D, Vértes Z, Könczöl F, Belagyi J. Thermal transitions ofactin. J Thermal Anal Calorim. 2009;95:713–9.
Türmer K, Könczöl F, Lőrinczy D, Belágyi J. AMP.PNP affects the dynamical properties of monomer and polymerized actin. a DSC and an EPR study. J Thermal Anal Calorim. 2012;108:95–100.
Farkas P, Szatmári D, Könczöl F, Lőrinczy D. Cyclophosphamide treatment evoked side effect on skeletal muscle actin, monitored by DSC. J Therm Anal Calorim. 2022;147:3609–14.
Torres K, Trębacz H, Chrościcki A, Pietrzyk Ł, Torres A. Evaluation of peritoneal tissue by means of differential scanning calorimetry (DSC). Folia Histochem Cytobiol. 2011;49:700–5.
Acknowledgements
This work was supported by CO-272 (OTKA) grant (D.L.).
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
Overall, after a previous physical separation of the intestinal wall structure into, among others, the muscle layer component, in this study we are able to detect and identify this layer for the first time by thermal transitions after deconvolution of the aggregated DSC curves by a mathematical method.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for publication
Copyright form has been uploaded with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferencz, A., Vértes, Z. & Lőrinczy, D. Deconvoluted DSC curves of intestinal muscle layer following warm and cold ischaemic injury. J Therm Anal Calorim 148, 831–836 (2023). https://doi.org/10.1007/s10973-022-11790-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11790-x