Abstract
Polymeric carbon nitride (PCN) was recently found to have extensive applications in the field of photocatalysis. Knowledge about thermal stability of PCN nanocomposites is crucial for this application and designing the final product. In this work, the thermal stability of PCN-Al2O3–ZrO2 nanocomposites was investigated. PCN nanocomposites were obtained in two steps: (1) microwave hydrothermal synthesis of co-precipitated AlOOH and ZrO2 precursors, followed by drying; (2) mixing the nanopowders with melamine powder and annealing in air in a tube furnace at 400, and 450 °C. The PCN nanocomposites were examined by attenuated total reflection technique of Fourier transformed infrared spectroscopy. Also, the evolved gas analysis was performed combining differential scanning calorimetry and thermogravimetry coupled with mass spectroscopy and FTIR. The results show that only PCN-Al2O3–ZrO2 nanocomposite obtained at 400 °C is stable from room temperature up to 490 °C and during thermal decomposition, in one step releases ammonia (NH3), cyanic acid (HNCO), water (H2O), and carbon dioxide (CO2). The limitation of the PCN-Al2O3–ZrO2 thermal stability and performance is AlOOH–ZrO2 used as a nanocomposite component.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Recent research of scientists from different scientific fields are focused on manufacturing composites and heterojunctions containing carbon nitride called by graphitic carbon nitride (g-C3N4) [1], melon or polymeric carbon nitride (PCN) [2]. This material is usually obtained from the pyrolysis of urea, cyanamide, thiourea, and melamine [2]. PCN is characterized by optical bandgap of 2.7 eV, possess a 2D crystal structure and great chemical stability [1]. Due to physical and chemical properties of PCN it was used in the fields of photocatalysis, electrochemistry and photo-electrochemistry. PCN based compounds have attracted attention due to their promising applications to clean up air and water pollution by degradation of organic pollutants.
It was discovered recently that by addition of a metal oxide (ZnO, ZrO2, TiO2, Al2O3, etc.) to PCN, it is possible to form a heterostructured composite. Such composite was characterized by long-term photostability, as well as thermal and mechanical stability [4, 5].
Analysis of literature shows that there is a lot of ambiguity as far as melamine thermal behavior. Synthesis of PCN from melamine was reported to occur in the broad temperature range from 400 to 700 °C [2, 6, 7]. During heating of melamine up to ~ 320 °C condensation reactions with evolution of NH3 take place. Above 360 °C during condensation of melamine and its reaction with residual cyanamide (NH2CN) melem (triaminotri‑s‑triazine, triamino-heptazine, C6N7(NH2)3) is formed. Melem was found to condense and form 1D chains of aminelinked heptazine units above 520 °C ([C6N7(NH2)(NH)]n—polymeric carbon nitride (PCN)) [2]. Melon, consist from layers made from 1D chains of NH-bridged melem (C6N7(NH2)3) monomers [8]. Praus et al. [6] recommend a higher than 600 °C temperature for PCN production from melamine. Authors [6] found, that at lower annealing temperature such as 550 °C and/or 575 °C PCN formation takes place, with the empirical formula C6N9H3. At higher temperature this material decomposes. Melamine annealed at 600 °C leads to C6N9H2 formation (which is very close to PCN) with the maximal C/N molar ratio of 0.68. This value indicates incomplete condensation of amino groups of PCN [6]. PCN is known as the most stable composition under ambient conditions among the family of carbon nitrides [2]. It was found [8] that the empirical PCN composition dependence on preparation method. Authors summarized that that melon structure is a mixture of molecules of different shapes and sizes. If so, PCN structure has rather amorphous character [8].
In previous work, it was demonstrated [7] that by using a tube furnace and applying the fast 50 °C/min heating rate during melamine and AlOOH-ZrO2 annealing favorable PCN formation can be achieved at 400 °C. This temperature of PCN-nanocomposite formation is lower than reported in literature [7]. It was postulated that lower PCN formation temperature was probably due to the interaction of the melamine with the AlOOH/γ-Al2O3 and ZrO2 nanoparticles (NPs). The presence of AlOOH/γ-Al2O3 and ZrO2 NPs provide high specific surface areas and morphology beneficial for the formation of a PCN layer. The PCN nanocomposite was characterized by a 3 eV band gap and showed significant photocatalytic ability for degradation of common pollutants.
Simultaneous thermal analysis is widely used in investigation of various nanocomposites thermal behavior [9,10,11,12,13]. DSC–TG–FTIR–QMS method is applied to identify gases released during the thermal treatment of different materials [9, 10, 13, 14]. In the literature there is lack of complete studies about thermal decomposition of PCN or PCN-nanocomposites with analysis of released gases. Miller et al. [12] reported that during thermogravimetric analysis NH3 is evolved from precursors such as melamine above approximately 450 °C, with C2N2 and CxNyHz species. However, detailed analysis of PCN-materials using EGA have not been conducted. Systematic and complementary DSC–TG–QMS–FTIR experiments performed for PCN-nanocomposites provide knowledge about PCN based materials which will be crucial for their synthesis performance investigation and their further application. Therefore, the aim of the work is to (1) find optimum synthesis conditions for PCN-Al2O3–ZrO2 nanocomposite photocatalysts; to (2) investigate the type of emitted gases during temperature program; and to (3) gain knowledge about interdependency between nanocomposite components.
Materials and methods
The microwave synthesis of nanopowder mixtures containing boehmite (AlOOH) with 20 mass% of ZrO2 is described in details in previous works [7, 9, 10]. The PCN nanocomposites were made by hand mixing of AlOOH-20 mass%ZrO2 nanopowder in zirconia mortar with 20 mass% of melamine (Sigma-Aldrich, CAS Number 108–78-1 (99%)). Further, prepared powder was annealed in a tube furnace in two temperatures: 400 °C and 450 °C for 5 h, in air. The heating rate applied was 50 °C min−1.
The surface morphology of the investigated materials was observed using a scanning electron microscope (Zeiss, model Ultra Plus, Zeiss, Oberkochen, Germany).
All powders after synthesis were examined by a Fourier transform infrared spectrometer (Bruker Optics, Tensor 27, Bruker BioSpin GmbH, Rheinstetten, Germany) equipped with ATR accessory and gas cell connected to STA device. The ATR–FTIR spectra were recorded in the 4000–400 cm−1 range, at room temperature. The resolution of spectra and their accuracy were as follows: 4 cm−1 and 1 cm−1, respectively.
Thermal stability of PCN-nanocomposites was investigated using DSC–TG–QMS–FTIR set of devices. The experiments were performed on a STA 449 F1 Jupiter by Netzsch equipped with SiC furnace. All tests were carried out with a heating rate of 10 °C min−1 from room temperature up to 1000 °C, with constant flow of air (60 mL min−1). The released gases during thermal heating of PCN-nanocomposites were detected by a mass spectrometer (QMS 403C Aeolos, Netzsch) and FTIR (Bruker Optics, Tensor 27,) coupled in line with the STA instrument. In order to avoid condensation of eventual melamine inside the equipment, maximum 20 mg of powder was placed into crucible for each measurement.
Results
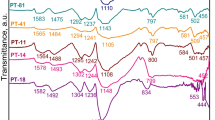
Figure 1 shows FTIR–ATR spectra of pure as-synthesized mixture of AlOOH and 20 mass% ZrO2 nanopowder (further denoted as AlOOH-20 mass% ZrO2 nanopowder, which will be treated as reference sample), as well as PCN-nanocomposites annealed at 400 and 450 °C. The reference material was described by author previously [7, 9] and is characterized by strong bands 3099, 3311 cm−1 which belong to asymmetric and symmetric O–H stretching vibrations from (O)Al–OH. Bands 1070, 1170 cm−1 are assigned to (HO)–Al=O asymmetric stretching and the O–H bending, respectively. The strong band at ~ 737 cm−1 can be attributed to Al–O–Al framework. AlOOH-20 mass% ZrO2 in melamine presence and after annealing at 400 and 450 °C does not show any visible vibrations associated to AlOOH, ZrO2 or Al2O3. In previous work [7] it was discussed already that PCN was identified only in the nanocomposite prepared at 400 °C. The confirmation of this fact was presence of PCN characteristic band at approximately 800 cm−1 [8]. It was found that -NH2 stretching vibrations of crystalline melamine (C3N3(NH2)3) at ~ 3475 and 3417 cm−1 are similar to spectrum for melem (C6N7(NH2)3), containing the C6N7 central heptazine unit [12].
Figure 2 shows morphology of obtained nanocomposites. Surface of the PCN nanocomposite obtained at 400 °C (Fig. 2a) looks like covered by thin organic layer what was discussed before in [7]. Nanocomposite obtained at 450 °C (Fig. 2b) represents morphology typical for Al2O3–ZrO2 nanoparticles mixture.
Figure 3 presents thermal behavior of as-synthesized AlOOH-20 mass%ZrO2, AlOOH-20 mass%ZrO2 annealed at 400 °C, and melamine. All presented results refer to PCN-nanocomposite thermal decomposition. Figure 3a shows thermal behavior (DSC–TG) of pure melamine which fully decomposes at approximately 350 °C, which is in agreement with literature [6, 15, 16]. Figure 3b shows thermal behavior for sample of pure AlOOH-20 mass% ZrO2 and transformation of AlOOH to Al2O3. The endothermic transition with peak at 300 °C can be associated to gibbsite transforming to χ-Al2O3 [17]. Further, exothermic peak at 410 °C can be explained by crystallization followed by boehmite (γ-AlOOH) transformation to γ-Al2O3 [17]. Tha all phase transformations are associated with OH− groups detachments. The total mass loss of this sample was 18 mass%. The phase transitions of AlOOH-ZrO2 materials in relation to annealing temperatures were discussed before [7, 9, 9, 18]. Thermal analysis results for AlOOH-20 mass%ZrO2 nanopowders annealed at 400 °C are presented at Fig. 3c, d. DSC curve shows exothermic event at ~ 230 °C (Fig. 3c) which is related to CO2 and H2O release (Fig. 3 d) and may be associated with crystallization process of γ-Al2O3. At 400 °C annealing temperature AlOOH should already transforms to Al2O3. However, it is clear that nano powder after thermal treatment at 400 °C still contains some AlOOH phase (boehmite or diaspore [17]). The broad endothermic transition with onset at 430 °C (Tpeak = 490 °C) is caused by further decomposition of AlOOH residues and phase transformation to alumina. The total mass loss for this material was 11 mass% related mainly to OH− groups release.
The DSC–TG curves of PCN-Al2O3–ZrO2 nanocomposites prepared at 400 °C and 450 °C are presented in Fig. 4. Nanocomposite prepared at 400 °C is characterized by narrow exothermic event in the temperature range: form 480 to 550 °C, while the sample prepared at 450 °C shows broad exothermic peak in the range 400–600 °C. The temperature stability of the nanocomposite prepared at 450 °C is similar to the reference material (Fig. 3a). The total mass loss observed during thermal decomposition was 19 and 9.5% for nanocomposite annealed at 400 and 450 °C, respectively.
Figure 5 shows mass spectra collected during thermal decomposition of PCN-Al2O3–ZrO2 nanocomposites prepared at 400 °C and 450 °C. The evolved gases during analysis of nanocomposite prepared at 400 °C were following: H2 (m/Z = 2), NO2, (m/Z = 14, 16, 30, 46), NO (m/Z = 14, 30), N2O (m/Z = 12, 14, 16, 30, 45), NH3 (m/Z = 14, 15, 16, 17), H2O (m/Z = 17, 18), HNCO (m/Z = 30, 43), CO2 (m/Z = 12, 44) (Fig. 5a, b). Sample prepared at 450 °C during thermal decomposition released CN−/HCN (m/Z = 26, 27) in addition to the gases listed above and rather did not evolved NOx. It should be noticed, that material obtained by annealing of AlOOH–ZrO2 and melamine at 450 °C is less stable than the one annealed at 400 °C. Five different maxima can be distinguished (Fig. 5c, d): peaks at 94 °C and 375 °C where H2O and H2 are released, at 239 °C with H2 and NH3 evolution, and 425 °C with CO2 evolution. At 579 °C, only water was released from the nanocomposite.
Figure 6 illustrates the 3D FTIR spectra for the gases evolved during thermal decomposition of PCN-Al2O3–ZrO2 nanocomposites in DSC–TG–FTIR–QMS experiment. The time axis seen on the map is related to the change of temperature, which can be visible on the subtracted spectra. The main signals visible on FTIR maps belong to NH3 (930–960 cm−1) and CO2 release (~ 2300 cm−1) which is in agreement with literature [19]. On subtracted spectra from the maps the additional signals can be distinguished: CO (2250–2300 cm−1), hydrocarbons (2800–3100 cm−1), HNCO (2260 cm−1- only in the sample prepared at 400 °C), H2O/OH− (~ 3300 cm−1), N2O (2150 cm−1). It is important to note that the FTIR spectra not only provide the information about the species of the released gas, but also show the relative intensities of the released gases. The emission of ammonia (NH3) is confirmed by the presence of absorption bands at 3335, and 966 cm−1 [11]. The cyanic acid (HNCO) bands were found at 1095 and at 3474 [11].
Figure 7 presents scheme of the PCN nanocomposites thermal decomposition prepared based on results from DSC–TG–QMS–FTIR experiment. Upon TG values analysis it can be concluded that as-synthesized AlOOH–ZrO2 nanopowders loses 18 mass% of mass during thermal treatment, while this material annealed at 400 °C loses only 11 mass%. It may lead to conclusion that while AlOOH–ZrO2 is mixed with 20 mass% of melamine and annealed at 400 °C ~ 11 mass% mass loss may origin form AlOOH–ZrO2 and only ~ 8 mass% from PCN. This means, that final PCN-nanocomposite may contain only 8 mass% of PCN. It expected then, that nanocomposite produced at higher temperature (450 °C) will contain even less melamine condensation products then its counterpart prepared at 400 °C.
Discussion
It was shown recently [7] that the PCN-nanocomposite obtained at 400 °C has very promising photocatalytic properties. The temperature 400 °C was surprising for PCN formation since according to literature [6] melamine undergoes transition to PCN above 500 °C. In this case the PCN formation at 400 °C takes place due to the presence of nano-sized metal oxide in the nanocomposite and rapid heating (50 °C min−1). Additionally, in the heterogeneous surface processes that may not occur easily in a pure melamine used by others or in different experimental procedure [1]. As a result, the conversion temperatures of melamine to PCN presented in literature are much higher due to the need to overheat the system for a transformation to occur. It is postulated that in this work PCN formation from melamine takes place at or around the true transition temperature. It can also be assumed that the formation of PCN on the surface of the nanopowder may be accelerated by water release during the phase transition AlOOH to Al2O3.
Based on results presented in Fig. 3, it is clear that thermal stability of PCN-nanocomposite depends on the stability of AlOOH–ZrO2 which is present in nanoscale form. Annealing of AlOOH–ZrO2 with melamine at 400 °C causes several chemical reactions during that process. At 400 °C AlOOH–ZrO2 nanopowder undergoes already endothermic reaction due to water release (Fig. 3b), while melamine itself should already decompose (Fig. 3a). Thus, thermal stability of PCN-nanocomposite is the same as Al2O3–ZrO2 stability (while comparing Figs. 3c and 4a) with the maximum of peak at ~ 500 °C.
Nanocomposite prepared at 450 °C does not show any presence of PCN but various melamine decomposition products what was described before [7]. Also, the amount of melamine decomposition products is lower than in case of nanocomposite prepared at 400 °C. Once the DSC curve of AlOOH-ZrO2 is analyzed (Fig. 3b), it confirms lower stability of material in comparison to PCN-nanocomposite obtained at 400 °C. At 450 °C, AlOOH–ZrO2 water released already (~ 300 °C, Fig. 3b) and material started crystallizing to form γ-Al2O3. It appears that exceeding crystallization temperature of Al2O3 in AlOOH–ZrO2 nanopowder influences dramatically PCN formation on Al2O3–ZrO2 surface.
The annealing of the mixture containing AlOOH, ZrO2 and melamine at 400 °C leads to a rather rapid process of bubbling off excess melamine, NH3, NOx and H2. In the same time, each solid particle of ZrO2 and AlOOH/Al2O3 act as nucleation centers for this bubbling process [7]. It is possible that, smaller and sharper particles may facilitate controlled interconversion of melamine at lower temperature and creating PCN layer around AlOOH and ZrO2 particles. Evolved gas analysis showed different thermal stability of investigated materials. Nanocomposite prepared at 450 °C starts gradually decomposing already at 94 °C, while PCN-nanocomposite obtained at 400 °C is stable from room temperature up to ~ 490 °C (when the final transformation AlOOH to Al2O3 takes place). Decomposition of this material causes following gases release: ammonia (NH3), water (H2O), cyanic acid (HNCO), carbon dioxide (CO2), nitric oxides (NO, NO2, N2O). These findings are in agreement with Praus et al. [6] who performed elemental analysis of the melamine condensation products at different temperatures. They showed [6] that all melamine condensation products contain nitrogen and hydrogen.
The evolution of gaseous carbon-containing species may indicate the fact that PCN does not exist in ideal C3N4 stoichiometry, which could be assumed as result when NH3 component will be removed from the N-rich compound. Instead, the stoichiometry of PCN materials seem to be close to C2N3H, corresponding to melon (C6N7(NH)(NH2)) or hydromelonic acid (C6N7(NCNH)3) [12].
Conclusions
The thermal stability of PCN-nanocomposites was studied for the first time using the DSC–TG–FTIR–QMS method. Designed experiments allowed confirming optimum synthesis conditions for PCN-Al2O3–ZrO2 nanocomposite photocatalysts. PCN-nanocomposite obtained at 400 °C was found to be thermally stable from room temperature up to ~ 490 °C. At 500 °C, full decomposition of the material takes place with evolution of ammonia (NH3), water (H2O), cyanic acid (HNCO), nitric oxides (NO, NO2, N2O).
In contrast to 400 °C route, nanocomposite prepared at 450 °C was thermally unstable with gradual decomposition starting already below 100 °C. The main difference in the type of emitted gases during thermal decomposition of the investigated nanocomposites was the presence of HCN/CN−. This compound was released in addition to NH3, H2, H2O, and HNCO in the case of nanocomposite obtained at 450 °C. The presence of HCN/CN− indicates poor stability of the nanocomposite produced at 450 °C.
The obtained results allow to conclude that in case of PCN-nanocomposites usage of AlOOH–ZrO2 as a composite components mixture play a major role. The results showed, that obtained in microwave synthesis AlOOH–ZrO2 uniformly mixed nanopowder undergoes several transitions of AlOOH to Al2O3. The final phase transition at 490 °C leads to complete decomposition of PCN. Even being close to this temperature (like in a case of nanocomposite annealed at 450 °C) is not beneficial for further thermal stability and performance of the nanocomposite. This could be explained by changing of morphology and internal rearrangements of nanoparticles leading to breaking continuity of PCN layer on Al2O3–ZrO2.
In the future, the mechanism of the PCN-nanocomposite formation will be investigated. However, currently it can be assumed that the formation of PCN in the nanocomposite is initiated by the disintegration of AlOOH and Al2O3 formation at approximately 400 °C in the presence of AlOOH, ZrO2 and melamine mixture.
References
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M. Nat Mater. 2009;8:76–80.
Kessler FK, Zheng Y, Schwarz D, Merschjann C, Schnick W, Wang X, Bojdys MJ. Nature Rev Mater. 2017. https://doi.org/10.1038/natrevmats.2017.30.
Sano T, Tsutsui S, Koike K, Hirakawa T, Teramoto Y, Negishi N, Takeuchi K. Mater Chem A. 2013;1:6489–96.
Jo W, Natarajan TS. J Colloid Interface Sci. 2016;482:58–72.
Jo W, Selvam NCS. J Hazard Mater. 2015;299:462–70.
Praus P, Svoboda L, Ritz M, Troppov I, Sihor M, Kocí K. Mater Chem Phys. 2017;193:438–46.
Koltsov I, Wojnarowicz J, Nyga P, Smalc-Koziorowska J, Stelmakh S, Babyszko A, Morawski A, Lojkowski W. Molecules. 2019. https://doi.org/10.3390/molecules24050874.
Lotsch BV, Dçblinger M, Sehnert J, Seyfarth L, Senker J, Oeckler O, Schnick W. Chem Eur J. 2007. https://doi.org/10.1002/chem.200601759.
Koltsov I, Przesniak-Welenc M, Wojnarowicz J, Rogowska A, Mizeracki J, Malysa M, Kimmel G. J Therm Anal Calorim. 2017;131:2273–84.
Malka IE, Danelska A, Kimmel G. Mater Today Proc. 2016. https://doi.org/10.1016/j.matpr.2016.06.018.
Cheng H, Liu Q, Liu J, Sun B, Kang S, Frost RL. J Therm Anal Calorim. 2014;116:195–203.
Miller TS, Belen Jorge A, Suter TM, Sella A, Cora F. Phys Chem Chem Phys. 2017. https://doi.org/10.1039/C7CP02711G.
Alshehri AJ. Hazard Mater. 2012;200–8:199–200.
Madarasz J, Bombicz P, Okuya M, Kaneko S, Pokol G. J Anal Appl Pyrolysis. 2004;72(2):209–14.
Aditya T, Jana J, Pal A. Pal T. ACS Omega. 2018. https://doi.org/10.1021/acsomega.8b00968.
Jiang Z. Liu G. RSC Adv. 2015. https://doi.org/10.1039/c5ra14586d.
Xie Y, Kocaefe D, Kocaefe Y, Cheng J, Liu W. Nanoscale Res Lett. 2016. https://doi.org/10.1186/s11671-016-1472-z.
Koltsov I, Kimmel G, Stelmakh S, Sobczak K, Lojkowski W. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-42058-417.
Koltsov I, Smalc-Koziorowska J, Przesniak-Welenc M, Małysa M, Kimmel G, McGlynn J, Ganin A, Stelmakh S. Materials. 2018. https://doi.org/10.3390/ma11050829.
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M. Nat Mater. 2009. https://doi.org/10.1038/nmat2317.
Wang YS, Wu R, Yuan MZ. Appl Surf Sci. 2015;20:21. https://doi.org/10.1016/j.apsusc.2015.08.173.
Acknowledgements
I.K is indebted to Jan Mizeracki for help in SEM observations and lab tasks.
Funding
This experiments were funded by Polish National Science Centre Grant Number: UMO-2013/11/D/ST8/03429.The research subject was carried out with the use of equipment funded by the project CePT, reference: POIG.02.02.00-14-024/08, financed by the European Regional Development Fund within the Operational Programme “Innovative Economy” for 2007–2013.
Author information
Authors and Affiliations
Contributions
IK conceived and designed experiments, wrote the paper, synthesized materials, and performed DSC–TG–QS–FTIR and FTIR–ATR tests/analysis of results; this publication is part of Iwona Koltsov’s habilitation thesis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Samples of the all compounds described in this work are available from I.K.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koltsov, I. Thermal stability of polymeric carbon nitride (PCN)-Al2O3–ZrO2 nanocomposites used in photocatalysis. J Therm Anal Calorim 147, 7675–7682 (2022). https://doi.org/10.1007/s10973-021-11090-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11090-w