Abstract
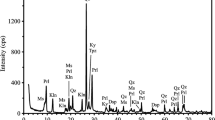
Thermal behaviour of shelly Estonian phosphorite ores from Iru, Toolse, Ülgase deposits and their concentrates have been studied. The phosphorus-bearing mineral in Estonian phosphate ore is fluorcarbonate apatite (francolite), originated from brachiopod Obolus apollinis shells that makes it different from all other sedimentary phosphate ores. The experiments on a Setaram Labsys Evo 1600 thermoanalyzer coupled with Pfeiffer Omnistar Mass Spectrometer for evolved gases analysis were carried out under non-isothermal condition at the heating rate of 10 °C min−1 up to 1200 °C in an oxidizing and inert atmosphere containing 79% of Ar and 21% of O2 or 100% Ar, respectively. The results obtained indicated the complicated character of transformations occurring at thermal treatment of Estonian phosphorites and certain differences depending on the mineralogical composition of sample and gaseous environment. The changes in francolite structure, probable substitution of sulphur additionally to carbonate, were studied by FTIR and XRD. The oxygen in gaseous atmosphere suppresses the liberation of carbonate and sulphate from the structure of francolite. The character of CO2 and SO2 release at heating depending on the atmosphere composition was explained. The impact of thermal treatment of phosphorite on the P2O5, CaO and SO−24 solubility in 2% citric acid solution and on the particles pore volume, as well as their dependence on each other, was also studied.








Similar content being viewed by others
References
U.S.Geological Survay. Mineral commodity summaries 2018. Phosphate Rock. http://www.minerals.usgs.gov. Accessed Apr 2019.
Pufahl PK, Groat LA. Sedimentary and igneous phosphate deposits: formation and exploration: an invited paper. Econ Geol. 2017;112:483–516.
Cordell D, Drangert J-O, White S. The story of phosphorus: global food security and food for thought. Glob Environ Change. 2009;19:292–305.
North Africa: phosphates powerhouse. Regional report. Fertil Int. 2019;489:42–46. http://fertilizerinternational.com. Accessed Apr 2019.
Cordell D, White S. Sustainable phosphorus measures: strategies and technologies for achieving phosphorus security. Agronomy. 2013;3:86–116.
http://www.maeselts.ee/ulevaade-fosforiidist. Accessed Mar 2010.
El Asri S, Laghzizil A, Alaoui A, Saoiabi A, M’Hamdi R, El Abbassi K, Hakam A. Structure and thermal behaviors of Moroccan phosphate rock (Bengurir). J Therm Anal Calorim. 2009;95:15–9.
Laghzizil A, Elhrech N, Britel O, Bouhaouss A, Ferhat M. Removal of fluoride from Moroccan phosphate and synthetic fluorapatites. J Fluorine Chem. 2000;101:69–73.
Aouad A, Benchanâa M, Mokhlisse A, Ounas A. Thermal analysis of Moroccan phosphates “Youssoufia” in an oxidative atmosphere by TG and DSC. J Therm Anal Calorim. 2004;75:887–900.
Mgaidi A, Ben Brahim F, Oulahna D, Nzihou A, El Maaoui M. Chemical and structural changes of raw phosphate during heat treatment. High Temp Mater Proc. 2004;23:185–94.
Elgharbi S, Horchani-Naifer K, Férid M. Investigation of the structural and mineralogical changes of Tunisian phosphorite during calcination. J Therm Anal Calorim. 2015;119:265–71.
Bachouâ H, Othmani M, Coppel Y, Fatteh N, Debbabi M, Badraoui B. Structural and thermal investigations of a Tunisian natural phosphate rock. J Mater Environ Sci. 2014;5:1152–9.
Galai H, Sliman F. Mineral characterization of the Oum El Khacheb phosphorites (Gafsa-Metlaoui basin; S Tunisia). Arab J Chem. 214. In press, corrected proofs available online 24 October 2014.
Knubovets R, Nathan Y, Shoval S, Rabinowitz J. Thermal transformation in phosphorites. J Therm Anal Calorim. 1997;50:229–39.
Nemliher J, Kurvits T, Kallaste T, Puura I. Apatite varieties in the shell of the Cambrian lingulate brachiopod Obolus apollinis Eichwald. Pros Estonian Acad Sci Geol. 2004;53:246–56.
Nemliher J. A new type of shell structure in a phosphatic brachiopod from the Cambrian of Estonia. Pros Estonian Acad Sci Geol. 2006;55:259–68.
Veiderma M. Phosphorite—vital resource for Estonia. Phosphorus Potassium. 1993;185:31–2.
Kaljuvee T, Veiderma M, Tõnsuaadu K, Vilbok H. Physico-chemical transformations during heating of phosphorites. J Therm Anal. 1988;33:839–44.
Veiderma M, Kaljuvee T, Knubovets R, Põldme M, Tõnsuaadu K. Thermal transformations in systems based on natural apatites. Phosphorus Sulfur Silicon. 1990;52:125–9.
Veiderma M. Studies on thermochemistry and thermal processing of apatites. Proc Estonian Acad Sci Chem. 2000;49:5–18.
Schröder JJ, Smit AL, Cordell D, Rosemarin A. Improved phosphorus use efficiency in agriculture: a key requirement for its sustainable use. Chemosphere. 2011;84:822–31.
Gaxiola RA, Edwards M, Elser JJ. A transgenic approach to enhance phosphorus use efficiency in crops as part of a comprehensive strategy for sustainable agriculture. Chemosphere. 2011;84:840–5.
Lu C, Tian H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance. Earth Syst Sci Data. 2017;9:181–92.
Taylor JC. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991;6:2–9.
Ward CR, Taylor JC, Cohen DR. Quantitative mineralogy of sandstones by X-ray diffractrometry and normative analysis. J Sediment GeoSci. 1999;69:1050–62.
Method 3.1.3. Extraction of the phosphorus soluble in 2% citric acid. Official Journal of the European Union. 21. 11. 2003;123–24.
Fan H, Song B, Li Q. Thermal behavior of goethite during transformation to hematite. Mater Chem Phys. 2006;98:148–53.
Diko M, Ekosse G, Ogola J. Fourier transform infrared spectroscopy and thermal analyses of kaolinitic clays from South Africa and Cameroon. Acta Geodyn Geomater. 2016;13:149–58.
Zhang M, Redfern SAT, Salje EKH, Carpenter MA, Hayward CL. Thermal behavior of vibrational phonons and hydroxyls of muscovita in dehydroxylation: in situ high-temperature infrared spectroscopic investigations. Am Mineral. 2010;95:1447–57.
Paulik F, Paulik J, Arnold M. Kinetics and mechanism of decomposition of pyrite under conventional and quasi-isothermal–quasi-isobaric thermoanalytical conditions. J Therm Anal Calorim. 1982;25:313–25.
Pelovski Y, Petkova V. Investigation on thermal decomposition of pyrite. Part I. J Therm Anal Calorim. 1999;56:95–9.
Hu G, Dam-Johansen K, Wedel S, Hansen JP. Decomposition and oxidation of pyrite. Prog Energy Combust Sci. 2006;32:295–314.
Põldme M, Põldme J, Utsal K, Kirs J. Thermal behavior of Estonian pyritic phosphorites. J Inorg Chem. 1985;30:877–81 (in Russian).
Petkova V, Koleva V, Kostova Sarov S. Structural and thermal transformations on high energy milling of natural apatite. J Therm Anal. 2015;121:217–25.
Baumer A, Caruba R, Ganteaume M. Carbonate-fluorapatite: mise en évidence de la substitution 2PO43− → SiO44− + SO42− par spectrométrie infrarouge. Eur J Mineral. 1990;2:297–304.
Antonakos A, Liarokapis E, Leventouri T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials. 2007;28:3043–54.
Koleva V, Petkova V. IR spectroscopic study of high energy activated Tunisian phosphorite. Vib Spectosc. 2012;58:125–32.
Madejová J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1–10.
Eisazadeh A, Kassim KA, Nur H. Solid-state NMR and FTIR studies of lime stabilized montmorillonitic and lateritic clays. Appl Clay Sci. 2012;67–68:5–10.
Alver BE, Dikmen G, Alver Ö. Investigation of the influence of heat treatment on the structural properties of illite-rich clay mineral using FT-IR, 29Si MAS NMR, TG and DTA methods. Anadolu Univ J Sci Technol A Appl Sci Eng. 2016;17(5):823–9.
Smith DH, Seshadri KS. Infrared spectra of Mg2Ca(SO4)3, MgSO4, hexagonal CaSO4 and orthorhombic CaSO4. Spectrochim Acta, Part A. 1999;55:795–805.
Tõnsuaadu K, Peld M, Quarton M, Bender V, Veiderma M. Studies on SO42− ion incorporation into apatite structure. Phosphorus Sulfur Silicon. 2002;177:1873–6.
Veiderma M, Tõnsuaadu K, Knubovets R, Peld M. Impact of anion substitutions on apatite structure and properties. J Organometall Chem. 2005;690:2638–43.
Tran LK, Stepien K, Yoder C. Substitution of sulfate in apatite. Am Mineral. 2017;102:1971–6.
Acknowledgements
This study was supported by Institutional Research Funding (IUT33-19) of the Estonian Ministry of Education and Research and by Research Grant RITA1/01-01-11 (LEP 17096).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaljuvee, T., Tõnsuaadu, K., Traksmaa, R. et al. Thermal behaviour of Estonian phosphorites from different deposits. J Therm Anal Calorim 142, 437–449 (2020). https://doi.org/10.1007/s10973-019-09056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09056-0