Abstract
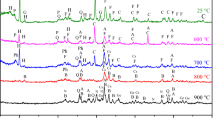
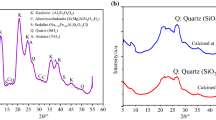
Rock phosphate is the fundamental component for the manufacture of phosphoric acid and phosphate fertilizers. The aim of this study is to predict how sample preparation of Kef Essennoun phosphate responds to heat treatment and to estimate the purity of the raw and calcined phosphates. The influence of temperature (600–900 °C) was evaluated. The evolution of thermal treatment and the characterization of the Kef Essennoun ore (Djebel Onk mine, Algeria) were carried out by various analytical techniques including X-ray diffraction (XRD), wavelength-dispersive X-ray fluorescence, Fourier-transform infrared spectroscopy, thermogravimetric analysis/differential thermal analysis, and scanning electron microscopy (SEM). The results showed that the raw phosphate is a mixture of six phases, of which carbonate hydroxyapatite [Ca10(PO4)3(CO3)3(OH)2] is the dominant phase. With heat treatment, at 700 °C, a fluorapatite [Ca5(PO4)3F] phase appeared, as confirmed by XRD analysis, with good crystallization indicated by SEM. The P2O5 content was increased from 28.389% in raw phosphate to 31.085% in the calcined product. The dissolution of the calcined phosphate at 900 °C was completed by HNO3 acid attack, and occurred rapidly at ambient temperature. The results show that the production of phosphoric acid by Ca5(PO4)3F was more easily achieved with optimized consumption of the acid attack.











Similar content being viewed by others
References
Slansky M. Géologie des phosphates sédimentaires. Mém BRGM. 1980;114:92.
Biswas DR, Narayanasamy G. Rock phosphate enriched compost: an approach to improve low-grade Indian rock phosphate. Biores Technol. 2006;97:2243–51.
Sugiyama S. Approach using apatite to studies on energy and environment. Phosphorus Res Bull. 2007;21:1–8.
Blazy P, Jdid EA. Décarbonisation des phosphates sédimentaires par calcination dynamique. C R Acad Sci Paris. 1995;321:287–94.
Bounemia L, Mellah A. Effects of initial concentration and irradiation dose to degradation of di butyl phthalate from phosphoric acid (30 % P2O5). Radiochim Acta. 2018. https://doi.org/10.1515/ract-2018-2984.
Khoshjavan S, Rezai B. Beneficiation of refractory rock phosphate by calcination and flotation. Miner Metall Explor. 2011;28(4):187–92.
Abouzeid AZM, El Jallad IS, Orphy MK. Calcareous phosphates and their calcined product. Miner Sci Eng. 1980;12:3–83.
Becker P. Phosphate and phosphoric acid. In: Chapter 2, Process review. Marcel Dekker Inc; 1989. pp. 35–140.
Blazy P, Samama J-C. Évolution duCO2 lors de la calcination d’un phosphate apatitique suivie d’une carbonatation par CO2 gazeux. Earth Planet Sci. 2001;333:271–6.
Sis H, Chander S. Reagents used in the flotation of phosphate ores: a critical review. Miner Eng. 2003;16(7):577–85.
Malek N. Influence de la composition chimique et minéralogique du phosphate noirdu gisement de Djebel Onk sur le procédé de traitement. Ph. D. thesis, Université AMira de Bejaïa, Algeria; 2007.
Özer AK. The characteristics of phosphate rock for upgrading in a fluidized bed. Adv Powder Technol. 2003;14:33–42.
Merabet D, Benabdeslam N, Bezzi N, Ikhlef T, Arkoub H. Influence de la composition chimique et minéralogique du phosphate noir du gisement de Djebel Onk(Tébessa) sur le procédé de traitement. Ann Chim Sci Mater. 2004;29(5):69–85.
Abu-Eishah SI, El-Jallad IS, MuthakerM Touqan M, Sadeddin W. Beneficiation of calcareous phosphate rocks using dilute acetic acid solutions: optimisation of operating conditions for Ruseifa (Jordan) phosphate. Int J Miner Process. 1991;31:115–26.
Straaten PV. Rocks for crops: agrominerals of sub-Saharan Africa. Guelph: ICRAF; 2002.
Legeros RZ. Apatite crystallites: effects of carbonate on morphology. Science. 1967;155:1409.
Sakae T. Variations in dental enamel crystallites and micro-structure. J Oral Biosci. 2006;48(2):85–93.
Authier A. Early days of X-ray crystallography, OXFORD, ISBN 978-0-19-965984-5; (2013).
Hassanzadeh-Tabrizi SA. Low temperature synthesis and luminescence properties of YAG: Eunano powders prepared by modified sol-gel method. Trans Nonferrous Met Soc China. 2011;21:2443–7.
Leroy N, Bres E. Structure and substitutions in fluorapatite. Eur Cells Mater. 2001;2:36–48.
Silverstein M, Basler C, Morrill G. Identification spectrométrique des composés organiques. 2nd ed. Bruxelles: Deboock; 2007.
Rouessac F, Rouessac A. Analyse chimique. Méthodes et techniques modernes. 6th ed. Paris: Dunod; 2004.
Bezzi N, Aïfa T, Hamoudi S, Merabet D. Trace elements of Kef Es Sennoun natural phosphate (Djebel Onk, Algeria) and how they affect the various mineralurgic modes of treatment. Procedia Eng. 2012;42:1915–27.
Bonel G. Contribution à l’étude de la carbonatation des apatites. Ann Chim. 1972;7:127–44.
Heydarpour T, Rezai B, Gharabaghi MA. Kinetics study of the leaching of a calcareous phosphate rock by lactic acid. Chem Eng Res. 2011;89:2153–8.
Kunii D, Levenspiel O. Fluidization engineering. New York: Wiley; 1969. p. 1–63.
Afnor NF. Sols: reconnaissance et essais–Détermination de la teneur en carbonate–Méthode du calcimètre. 1996;11:94–048.
Belboom S, Szöcs C, Léonard A. Environmental impacts of phosphoric acid production using di-hemihydrate process: a Belgian case study. J Clean Prod. 2015;108:978–86.
Bisutti I, Hilke I, Schumacher J, Raessler M. A novel single dual temperature combustion method for the determination of organic, inorganic and total carbon in soil samples. Talanta. 2007;71:521–8.
Aouadi-Selmi H, Antar K, Khattech I. Thermochemical and kinetic study of the attack of fluorapatite by sulfuric acid solution at different temperatures. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-09044-4.
Soussi-Baatout A, Ibrahim K, Khattech I, Jemal M. Attack of Tunisian phosphate ore by phosphoric acid: kinetic study by means of differential reaction calorimetry. J Therm Anal Calorim. 2016;124:1671–8.
Acknowledgements
The authors are grateful for the financial support of this project by the Nuclear Research Center of Algiers (CRNA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bounemia, L., Mellah, A. Characterization of crude and calcined phosphates of Kef Essennoun (Djebel Onk, Algeria). J Therm Anal Calorim 146, 2049–2057 (2021). https://doi.org/10.1007/s10973-020-10167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10167-2