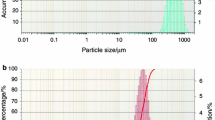
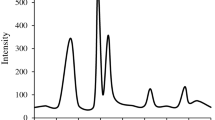
Abstract
The effect of mass ratio of fuel to oxidant in pyrotechnic compositions of Mg/Ba(NO3)2 and Mg/Sr(NO3)2 was studied using the non-isothermal thermogravimetry (TG) and differential scanning calorimetry (DSC) techniques. The mass ratios 10:90, 25:75 and 50:50 of Mg powder to oxidant (nitrate salt) were used for preparation of pyrotechnic compositions. The kinetic parameters of thermal ignition reactions of pyrotechnics were obtained by using TG/DSC curves under nitrogen atmosphere at heating rates of 20, 25 and 30 °C min−1. The DSC curves showed that the 25:75 weight ratio of fuel to oxidant resulted in a complete ignition reaction and showed the highest enthalpy of the reaction. Also, this mass ratio was not indicated a mass gain after the combustion step in the TG curves. The model-free methods of Kissinger, Ozawa–Flynn–Wall (OFW) and Kissinger–Akahira–Sunose (KAS) were used for calculation of activation energy (Ea) of ignition reactions of pyrotechnics. The Ea values 130.5 and 146.3 by Kissinger, 138.3 and 153.2 by OFW and 132.2 ± 2.4 and 146.3 ± 1.7 kJ mol−1 by KAS methods, respectively, were obtained for Mg/Ba(NO3)2 and Mg/Sr(NO3)2 pyrotechnics. Also, the nonlinear model-fitting method was used to determine the pre-exponential factor (A) and kinetic model function. The sigmoidal shapes were resulted from the curves of conversion factor (α) versus T. The model of A3/2 with the functions of g(α) = [− ln(1 − α)]2/3 and f(α) = 3/2(1 − α)[− ln(1 − α)]1/3 as a nucleation reaction model was proved by using the model-fitting method for the ignition reaction of both pyrotechnic compositions. The ln A values 8.1 and 7.6 min−1 were obtained, respectively, for the ignition reaction of magnesium powder with Ba(NO3)2 and Sr(NO3)2.







Similar content being viewed by others
References
Conkling JA, Mocella C. Chemistry of pyrotechnics basic principles and theory. 2nd ed. London: Taylor and Francis; 2010.
Redkar AS, Mujumdar VA, Singh SN. Study on magnesium based pyrotechnic composition as a priming charge. Def Sci J. 1996;46:41–7.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244–51.
Tuukkanen I, Brown SD, Charsley EL, Goodall SJ, Rooney JJ, Griffiths TT, Lemmetyinen H. Studies on the ageing of a magnesium–strontium nitrate pyrotechnic composition using isothermal microcalorimetry and thermal analysis techniques. Thermochim Acta. 2004;417:223–9.
Tuukkanen IM, Charsley EL, Laye PG, Rooney JJ, Griffiths TT, Lemmetyinen H. Pyrotechnic and thermal studies on the magnesium–strontium nitrate pyrotechnic system. Propellants Explos Pyrotech. 2006;31:110–5.
Miao Y, Liping C, Jinyang YV, Jinhua P. Thermoanalytical investigation on pyrotechnic mixtures containing Mg–Al alloy powder and barium nitrate. Proc Eng. 2012;45:567–73.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Technol. 2008;180:2093–102.
Kang X, Zhang J, Zhang Q, Du K, Tang Y. Studies on ignition and afterburning processes of KClO4/Mg pyrotechnics heated in air. J Therm Anal Calorim. 2012;109:1333–40.
Zhu CG, Wang HZ, Min L. Ignition temperature of magnesium powder and pyrotechnic composition. J Energ Mater. 2014;32:219–26.
Pouretedal HR, Ravanbod M. Kinetic study of ignition of Mg/NaNO3 pyrotechnic using non-isothermal TG/DSC technique. J Therm Anal Calorim. 2015;119:2281–8.
Onem E, Yorgancioglu A, Karavana HA, Yilmaz O. Comparison of different tanning agents on the stabilization of collagen via differential scanning calorimetry. J Therm Anal Calorim. 2017;129:615–22.
Pouretedal HR, Ebadpour R. Application of non-isothermal thermogravimetric method to interpret the decomposition kinetics of NaNO3, KNO3, and KClO4. Int J Thermophys. 2014;35:942–51.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17:407–33.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochimca Acta. 1999;340–341:53–68.
Sinapour H, Damiri S, Pouretedal HR. The study of RDX impurity and wax effects on the thermal decomposition kinetics of HMX explosive using DSC/TG and accelerated aging methods. J Therm Anal Calorim. 2017;129:7–792.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
De Klerk WPC, Colpa W, Ekeren PJ. Ageing studies of magnesium–sodium nitrate pyrotechnic compositions. J Therm Anal Calorim. 2006;85:203–7.
Babar Z, Malik A. Thermal decomposition, ignition and kinetic evaluation of magnesium and aluminium fuelled pyrotechnic compositions. Centr Eur J Energ Mater. 2015;12:579–92.
Tuukkanen IM, Charsley EL, Goodall SJ, Laye PG, Rooney JJ, Griffiths TT, Lemmetyinen H. An investigation of strontium nitrite and its role in the ageing of the magnesium–strontium nitrate pyrotechnic system using isothermal microcalorimetry and thermal analysis techniques. Thermochim Acta. 2006;443:116–21.
Brown SD, Charsley EL, Goodall SJ, Laye PG, Rooney JJ, Griffiths TT. Studies on the ageing of a pyrotechnic composition using isothermal heat flow calorimetry and thermal analysis techniques. Thermochim Acta. 2003;401:53–61.
Pouretedal HR, Damiri S, Ghaemi EF. Non-isothermal studies on the thermal decomposition of C4 explosive using the TG/DTA technique. Centr Eur J Energ Mater. 2014;11:285–94.
Musanic SM, Houra IF, Suceska M. Applicability of non-isothermal DSC and Ozawa method for studying kinetics of double base propellant decomposition. Centr Eur J Energ Mater. 2010;7:233–51.
Jankovic B, Mentus S, Jankovic M. A kinetic study of the thermal decomposition process of potassium metabisulfite: estimation of distributed reactivity model. J Phys Chem Solids. 2008;69:1923–33.
Edreisa EMA, Yao H. Kinetic thermal behaviour and evaluation of physical structure of sugar cane bagasse char during non-isothermal steam gasification. J Mater Res Technol. 2016;5:317–26.
Wang PC, Xie Q, Xu YG, Wang JQ, Lin QH, Lu M. A kinetic investigation of thermal decomposition of 1,1′-dihydroxy-5,5′-bitetrazole-based metal salts. J Therm Anal Calorim. 2017;130:1213–20.
Lima ACR, China BLF, Jawada ZA, Hiia KL. Kinetic analysis of rice husk pyrolysis using Kissinger–Akahira–Sunose (KAS) method. Proc Eng. 2016;148:1247–51.
Akbar J, Iqbal MS, Massey S, Masih R. Kinetics and mechanism of thermal degradation of pentose- and hexose-based carbohydrate polymers. Carbohydr Polym. 2012;90:1386–93.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2017;127:1751–6.
Nowak M, Cichy B, Kużdżał E. Kinetics of melamine phosphate thermal decomposition in DSC studies. J Therm Anal Calorim. 2016;126:277–85.
Noisong P, Danvirutai C. Kinetics and mechanism of thermal dehydration of KMnPO4·H2O in a nitrogen atmosphere. Ind Eng Chem Res. 2010;49:3146–51.
Zhao FQ, Rong-Zu H, Chen P, Luo Y, Gao SL, Song J-R, Shi QZ. Kinetics and mechanism of the exothermic first-stage decomposition reaction of dinitroglycoluril. Chin J Chem. 2004;22:649–52.
Ravanbod M, Pouretedal HR, Amini MK, Ebadpour R. Kinetic study of the thermal decomposition of potassium chlorate using the non-isothermal TG/DSC technique. Centr Eur J Energ Mater. 2016;13:261–70.
Liu J, Song D, Guan H. Isothermal kinetics approach to investigating the oxidation process of red phosphorus in air. J Therm Anal Calorim. 2017;128:1801–10.
Acknowledgements
We would like to thank the research committee of Malek-Ashtar University of Technology (MUT), M. Shahmoradi, A. Zareh and S. Sattari for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouretedal, H.R., Loh Mousavi, S. Study of the ratio of fuel to oxidant on the kinetic of ignition reaction of Mg/Ba(NO3)2 and Mg/Sr(NO3)2 pyrotechnics by non-isothermal TG/DSC technique. J Therm Anal Calorim 132, 1307–1315 (2018). https://doi.org/10.1007/s10973-018-7028-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7028-y