Abstract
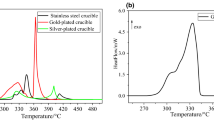
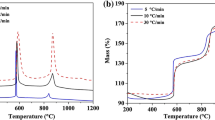
The isothermal oxidation process of red phosphorus (RP) was investigated by thermogravimetric analysis in the temperature range of 320–480 °C. The model-free, model-fitting and reduced-time plot methods were used to research the oxidation kinetics of RP. According to the dα/dt − α and model-free curves in different temperatures, the oxidation temperatures are divided into two ranges: lower temperatures (320–380 °C) and higher temperatures (390–410 °C). The apparent activation energy and oxidation controlling mechanisms in different temperature ranges are obtained. Besides, the trend of the oxidation rate with mass loss or temperature ranges is discussed.







Similar content being viewed by others
References
Toxicity of military smokes and obscurants; National Research Council; chapter no. 4, vol. 1. National Academy Press: Washington D.C. 1997. pp. 98–126.
Horold S. Improvements in stability of red phosphorus. Presented at the 27th international pyrotechnics seminar—special session on red phosphorus, Grand Junction, CO, 2000.
Kelly R. Subject: Solicitation letter to the USMC for the RP project trans atlantic consultancy LLC, 27 2 2006.
Edward NP. Flame-retardant thermoplastics. I. Polyethylene–red phosphorus. J Appl Polym Sci. 1979;24:1457–64.
Yeh JT, Hsieh SH, Cheng YC, Yang JM, Chen NK. Combustion and smoke emission properties of poly (ethylene terephthalate) filled with phosphorous and metallic oxides. Polym Degr Stab. 1998;61:399–407.
Levchik GF, Vorobyova SA, Gorbarenko VV, Levchik SV, Weil ED. Some mechanistic aspects of the fire retardant action of red phosphorus in aliphatic nylons. J Fire Sci. 2000;18:172–82.
Levchik SV, Weil ED. Combustion and fire retardancy of aliphatic nylons. Polym Int. 2000;49:1033–73.
Przybylski K, Brylewski T, Durda E, Gawel A, Kruk A. Oxidation properties of the Crofer 22APU steel coated with La0.6–Sr0.4Co0.2Fe0.8O3 for IT—SOFC interconnect applications. J Therm Anal Calorim. 2014;116(2):825–34.
Gyurov S, Rabadjieva D, Kovatcheka D, Kostova Y. Kinetics of copper slag oxidation under non isothermal conditions. J Therm Anal Calorim. 2014;116(2):945–53.
Guo W, Xiao H, Yasuda E, Cheng Y. Oxidation kinetics and mechanisms of a 2D-C/C composite. Carbon. 2006;44:3269–76.
Guo W, Xiao H. Mechanisms and modeling of oxidation of carbon felt/carbon composites. Carbon. 2007;45:1058–65.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 24 May 2016.
Ratusz K, Popis E, Ciemniewska-Zytkiewicz H, Wroniak M. Oxidative stability of camelina (Cameline sativa L.) oil using pressure differential scanning calorimetry and Rancimat method. J Therm Anal Calorim. 2016;126:343–51.
Jianshu Z, Xinmei H, Xiangbin W, Ye M, Xin Z, Lei Z. Isothermal oxidation mechanism of Nb-Ti-V-Al-Zr alloy at 700–1200 °C: diffusion and interface reaction. Corros Sci. 2015;96:186–95.
Gao PZ, Wang HJ, Jin ZH. Study of oxidation properties and decomposition kinetics of three-dimensional (3-D) braided carbon fiber. Thermochim Acta. 2004;414(1):59–63.
Tanaka H. Thermal analysis and kinetics of solid-state reactions. Thermochim Acta. 1995;267:29–44.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Brown M, Dollimore D, Galway A. Comprehensive chemical kinetics. Amsterdam: Elsevier; 1980.
Sharp JH, Brindley GW, Achar BNN. Numerical data for some commonly used solid state reaction equations. J Am Ceram Soc. 1966;49(7):379–82.
Halikia I, Neou-Syngouna P, Kolitsa D. Isothermal kinetic analysis of the thermal decomposition of magnesium hydroxide using thermogravimetric data. Thermochim Acta. 1998;320:75–88.
Luo RY, Cheng JW, Wang TM. Oxidation behavior and protection of carbon/carbon composites prepared using rapid directional diffused CVI techniques. Carbon. 2002;40(11):1965–72.
Vyazovkin S. Reply to ‘‘What is meant by the term ‘variable activation energy’ when applied in the kinetics analyses of solid state decompositions (crystolysis reactions)?’’. Thermochim Acta. 2003;397:269–71.
Vyazovkin S, Sbirrazzuoli N. Estimating the activating energy for non-isothermal crystallization of polymer melts. J Therm Anal. 2003;72(2):681–6.
Vyazovkin S. Two types of uncertainty in the values of activation energy. J Therm Anal. 2001;64(2):829–35.
Khawam A, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics I. Isothermal kinetic studies. Thermochim Acta. 2005;429(1):93–102.
Acknowledgements
This work was supported by the innovation fund of Nanjing University of Science and Technology (Grant No. AE03001).
Author information
Authors and Affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Song, D. & Guan, H. Isothermal kinetics approach to investigating the oxidation process of red phosphorus in air. J Therm Anal Calorim 128, 1801–1810 (2017). https://doi.org/10.1007/s10973-016-6073-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6073-7