Abstract
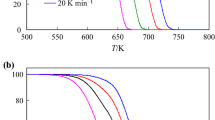
The non-isothermal thermogravimetric method was used to study the thermal decomposition of \(\hbox {KClO}_{4}, \hbox {KNO}_{3}\), and \(\hbox {NaNO}_{3}\) at heating rates of (5, 10, 15, and 20) \(\hbox {K}\cdot \hbox {min}^{-1}\). The activation energy of thermal decomposition reactions was computed by isoconversional methods of Ozawa–Flynn–Wall, Kissinger–Akahiro–Sunose, and Friedman equations. Also, the kinetic triplet of the thermal decomposition of salts was determined by the model-fitting method of the modified Coats–Redfern equation. The activation energies of \(\hbox {KClO}_{4}, \hbox {KNO}_{3}\), and \(\hbox {NaNO}_{3}\) of (293 to 307, 160 to 209, and 192 to 245) \(\hbox {kJ}\cdot \hbox {mol}^{-1}\), respectively, are obtained by non–isothermal isoconversional methods. The modified Coats and Redfern method showed that the most probable mechanism functions \(g(\alpha )\) of \([-\hbox {ln}(1 - \alpha )]^{1/3}\) (model A3: Arami–Erofeev equation) and \((1 - \alpha )^{-1}- 1\) (model F2: second order) can be used to predict the decomposition mechanisms of \(\hbox {KClO}_{4}\), \(\hbox {KNO}_{3}\), and \(\hbox {NaNO}_{3}\), respectively.






Similar content being viewed by others
References
J.-S. Lee, C.-K. Hsu, K.-S. Jaw, Thermochim. Acta 367, 381 (2001)
D. Seetharamacharyulu, R.M. Mallya, V.R. Pai Vernerneker, J. Thermal Anal. 22, 17 (1981)
H. Ellern, Modern Pyrotechnics (Chemical Publishing Co., Inc., New York, 1961)
S.D. Brown, E.L. Charsley, S.J. Goodall, P.G. Laye, J.J. Rooney, T.T. Griffiths, Thermochim. Acta 401, 53 (2003)
E.S. Freeman, J. Am. Chem. Soc. 79, 838 (1957)
T. Bauer, D. Laing, R. Tamme, Int. J. Thermophys. 33, 91 (2012)
H. Chen, N. Liu, J. Am. Ceram. Soc. 93, 548 (2010)
D. Dollimore, S. Lerdkanchanaporn, K.S. Alexander, Thermochim. Acta 290, 73 (1997)
X. Gao, D. Chen, D. Dollimore, Thermochim. Acta 223, 75 (1993)
K. Chrissafis, J. Therm. Anal. Calorim. 95, 273 (2009)
S. Hosseini, S. Pourmortazavi, S. Hajimirsadeghi, Combust. Flame 141, 322 (2005)
Y. Hoshino, T. Utsunomiya, O. Ade, Bull. Chem. Soc. Jpn. 54, 1385 (1981)
H.E. Kissinger, Anal. Chem. 29, 1702 (1957)
T. Ozawa, H. Isozaki, A. Negishi, Thermochim. Acta 1, 545 (1970)
J.H. Flynn, Thermochim. Acta 4, 323 (1966)
J.W. Park, H.P. Lee, H.T. Kim, K.O. Yoo, Polym. Degrad. Stab. 67, 535 (2000)
P.E. Fischer, C.S. Jou, S.S. Gokalgandhi, Ind. Eng. Chem. Res. 26, 1037 (1987)
R. Ebrahimi-Kahrizsangi, M.H. Abbasi, Trans. Nonferr. Met. Soc. China 18, 217 (2008)
L. Gavernet, M. Luisa Villalba, L. Bruno Blanch, I. Daniela Lick, Eur. J. Chem. 4, 44 (2013)
P. Noisong, C. Danvirutai, Ind. Eng. Chem. Res. 49, 3146 (2010)
N. Sbirrazzuoli, L. Vincent, A. Mija, N. Guigo, Chemom. Intell. Lab. Syst. 96, 219 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouretedal, H.R., Ebadpour, R. Application of Non-Isothermal Thermogravimetric Method to Interpret the Decomposition Kinetics of \(\hbox {NaNO}_{3}, \hbox {KNO}_{3}\), and \(\hbox {KClO}_{4}\) . Int J Thermophys 35, 942–951 (2014). https://doi.org/10.1007/s10765-014-1636-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-014-1636-y