Abstract
Monophasic polycrystalline samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution with limited homogeneity (0 < x ≤ 0.2222) and cationic vacancies (□) have been prepared by high-temperature annealing of PbMoO4/Pr2(WO4)3 mixtures composed of 40.00 mol% and less of praseodymium tungstate. Initial reactants and obtained ceramic materials were characterized by XRD, simultaneous DTA–TG, IR and UV–Vis–NIR techniques. The X-ray diffraction analysis showed that the monophasic samples crystallize in a tetragonal symmetry, with space group I4 1 /a (a scheelite-type structure), and PbMoO4 is a matrix of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution. Thermal stability of samples under study strongly depends on concentration of Pr3+ ions. The Pb0.9286□0.0238Pr0.0476(MoO4)0.9286(WO4)0.0714 solid solution (x = 0.0238) shows the highest melting point (1055 °C), and this value is slightly higher than the melting point of PbMoO4 (1040 °C). Lead molybdate and samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution are insulators having indirect band gap E g > 3 eV. The observed band gap of monophasic samples shows a nonlinear variation with a change of Pr3+ ions concentration in the scheelite framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Divalent metal molybdates and tungstates (AMO4, where M = Ca, Sr, Ba, Ca, Cd, and Pb; M = Mo or W) have wide industrial applications in many fields. They have been used as laser host materials, Raman lasers, optical fibers, humidity sensors, magnetic and photoluminescence materials, and catalysts [1–13]. Among them, lead molybdate and lead tungstate are very attractive materials because of their applications as acousto-optical modulators, deflectors, ion conductors, and solid-state scintillators for a nuclear instrumental application [14–22]. These materials doped with RE3+ ions exhibit good photoluminescence, and they are promising as laser hosts [14, 22]. Lead molybdate crystallizes with a scheelite-type tetragonal structure and has a space group (I4 1 /a), as well as four molecular formulas per unit cell (Z = 4) [1, 11, 15, 19, 20, 23, 24]. Its structure is made up of PbO8 dodecahedra and MoO4 tetrahedra connected via common vertices (Fig. 1). PbO8 polyhedra are connected via common edges and form a 3D framework (Fig. 1) [11, 19].
Our earlier studies on a reactivity in a solid state of divalent metal molybdates or tungstates (CdMoO4, CdWO4 and others) with rare-earth compounds (molybdates or tungstates) have been showed an existence of new interesting materials for applications in optoelectronics [25–34]. The scheelite-type ceramic materials, i.e., Cd0.25RE0.50□0.25WO4 (RE = Pr, Nd, Sm, Eu, and Gd, vacancy is denoted by □), were synthesized via a solid-state reaction of CdWO4 with rare-earth ditungstates RE2W2O9 [25–28]. In the scheelite Cd0.25Gd0.50□0.25WO4 host, where trivalent europium ions occupy only one low symmetry site, a very strong red emission was observed [25]. This Eu3+-doped cadmium and gadolinium tungstate proved to be a new promising polycrystalline red phosphor for WLEDs [25]. As compared to optical parameters of YAG:Nd, new cadmium and gadolinium tungstate doped with Nd3+ ions (Cd0.25Gd0.50□0.25WO4:Nd3+, the concentration of active ions 5 mol%) showed larger absorption cross section (2.5·10−19 cm2 at λ = 805 nm) and much higher emission at 1064 nm [26]. The broad spectral emission band observed in Cd0.25Gd0.50□0.25WO4:Nd3+ allows tuning of laser action over the 1030- to 1080-nm range for generation of very short pulses, and therefore, it has applications in pico- or femtosecond lasers [26]. Important group of very good and new candidates for applications in optoelectronics are scheelite-type cadmium and rare-earth metal molybdates Cd1−3x□xRE2xMoO4, where RE = Pr–Yb [29–34]. Value of x parameter strongly depends on a radius of trivalent rare-earth ion, and it can reach a maximum value of 0.25 [29–34]. The substitution of divalent cadmium ions by RE3+ ones leads to formation of cationic vacancies (□) in CdMoO4 framework [29–34]. The appearance of vacancies results in some disorder of crystal lattice around RE3+ ions. This fact is manifested by an emission of broad bands associated with f–f transitions in RE3+ laser ions (e.g., Nd3+ or Yb3+), and this phenomenon could be used for ultrashort laser pulses [30, 31]. Single crystals of some Cd1−3x □ x RE2x MoO4 (RE = Gd, Dy) solid solutions with different concentrations of RE3+ ion have been successfully grown by the Czochralski method [35, 36]. Their structure, dielectric and magnetic properties, and FTIR and Raman spectra were recorded and discussed in detail [35, 36].
The aim of this work is to make a detailed study of new Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution that could be used as scintillator material in polycrystalline or single-crystal form. Thus, we present the results on a homogeneity range of the solid solution under study, its structure, and thermal and spectroscopic properties.
Experimental
Sample preparation
Lead molybdate (PbMoO4) and praseodymium tungstate (Pr2(WO4)3) were used as the starting materials. Lead molybdate was prepared by heating PbO with MoO3 (both with the purity 99.95 %, Alfa Aesar) mixed in an equimolar ratio, and in the following thermal conditions: 550 °C (6 h), 600 °C (6 h), 700 °C (6 h), 800 °C (12 h), 900 °C (12 h), and 950 °C (12 h). Praseodymium tungstate was obtained using a solid-state reaction method [37–45] and Pr6O11 with WO3 (both with the purity 99.95 %, Alfa Aesar) mixed in the 1:9 molar ratio according to the procedure used by us in a synthesis of other rare-earth tungstates, and under thermal conditions reported earlier [25–28, 37–39]. Mixtures of starting reactants with Pr2(WO4)3 content changing from 0.50 to 50.00 mol% were homogenized in an agate mortar. Their initial content is presented in Table 1. Next, they were heated in air, in ceramics crucibles, with 12-h annealing stages, and in the temperature range from 900 to 975 °C. After each heating stage, all samples were cooled slowly down to ambient temperature, and for better reactivity, ground in an agate mortar. After a final sintering stage, monophasic samples were examined by XRD, DTA-TG, IR, and UV–Vis–NIR techniques. Additionally, their density was measured.
Methods
Powder X-ray diffraction patterns were collected in the 10–80° 2Θ range with the step 0.013° on a diffractometer Empyrean II (PANalytical) using Cu Kα radiation (λ = 0.15418 nm). XRD patterns were analyzed by a HighScore Plus 4.0 software package, and lattice parameters were calculated using the least squares refinement procedure and a POWDER software [47]. Simultaneous DTA and TG measurements were carried out on a TA Instruments thermal analyzer (model SDT 2960) at the heating and cooling rate of 10 °C min−1, in air (the gas flow 110 mL h−1), and using alumina crucibles. The mass of each sample for DTA-TG measurements was ~30 mg. DTA and TG curves were recorded in the temperature range of 20–1100 °C. UV–Vis–NIR diffuse reflectance spectra were recorded at room temperature and in the wavelength range of 190–1000 nm using a JASCO V-670 spectrophotometer equipped with an integrating sphere. The reflectance measurements were carried out using scan step 0.5 nm and scan speed 400 nm/min. IR spectra of samples were collected in the 1500–200 cm−1 spectral range on a Specord M-80 spectrometer (Carl Zeiss Jena) using pellets with KBr. The density of samples was measured on a Quantachrome Instruments Ultrapycnometer (model Ultrapyc 1200 e) using nitrogen (99.99 %) as a pycnometric gas. The measurements were carried out in five repetitions using ~4 g of each sample for the test. The error determine a density was estimated as ±0.01 g cm−3.
Results and discussion
XRD and IR studies
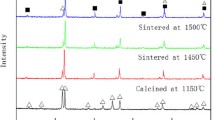
The X-ray powder diffraction patterns of initial reactants and all samples under study were recorded and analyzed. Figure 2 shows experimental XRD patterns of PbMoO4 and products obtained after the final sintering stage of some PbMoO4/Pr2(WO4)3 mixtures. It was observed that when the initial content of praseodymium tungstate was 40.00 mol% and less, the XRD patterns consisted of diffraction lines that could be attributed to a scheelite-type lattice. No additional peaks of other phases, e.g., initial reactants, have been observed. Additionally, all observed diffraction peaks shift toward higher 2θ angle with an increase in Pr2(WO4)3 content in initial mixtures. Very sharp and strong peaks clearly confirm good crystallinity of the as-prepared samples. All diffractions lines observed in the XRD patterns of single-phase materials were successfully indexed with a tetragonal symmetry and space group I4 1 /a. This fact has indicated a formation of a new solid solution and its synthesis can be described by the following equation:
A location of this solution is marked in the tetrahedron of PbO–WO3–Pr2O3–MoO3 system (Fig. 3). The lattice constants calculated by cell refinement fitting on the basis of XRD data for an adequate Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution identified in monophasic samples (Nos. 2–10), as well as experimental and calculated values of density are presented in Table 1. The cell parameters gradually decrease with an increase in value of x parameter only up to 0.2222 suggesting the solid solution limit has been achieved. Both, unit cell parameters and volume calculated for single-phase samples fulfill the Vegard law, i.e., are nearly linear function of x (Fig. 4). The diffraction pattern of a sample comprising initially 50.00 mol% of Pr2(WO4)3 consisted of diffraction lines due to Pb0.3334□0.2222Pr0.4444(MoO4)0.3334(WO4)0.6666 (the saturated solid solution), and additionally, the peaks corresponding to praseodymium tungstate have appeared. The above observations indicate that the solubility limit of Pr2(WO4)3 in a scheelite-type framework of PbMoO4 is not higher than 40.00 mol% and Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution exists for 0 < x ≤ 0.2222. Moreover, the results have indicated that Pr3+ (CN (coordination number) = 8, ionic radius −112.6 pm [46]) and W6+ (CN = 4, 42 pm [46]) ions were introduced to PbMoO4 matrix instead of Pb2+ (CN = 8, 129 pm [46]) and Mo6+ (CN = 4, 41 pm [46]) ions, respectively. Compensation of excess positive charge resulting from substitution of divalent ions by trivalent ones is accomplished by an appearance of cationic vacancies denoted as □.
Figure 5 shows IR spectra recorded for polycrystalline samples of PbMoO4 and Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x with different concentration of Pr3+ ions. For solid molybdates with a scheelite-type structure, the frequencies active in IR are observed in the wave number ranges 900–700 cm−1 (the stretching multiples ν 1 and ν 3) and 450–250 cm−1 (the bending modes ν 2 and ν 4) [33, 34, 48, 49]. Very narrow absorption bands can be observed in the IR spectrum of PbMoO4 (pure matrix). The first group of bands sequentially recorded at 853, 816, and 768 cm−1 can be assigned to the stretching modes of Mo–O bonds in MoO4 tetrahedra [33, 34, 48, 49]. On the other hand, the absorption bands with their maxima at 380 and 296 cm−1 can be related to the bending modes of Mo–O bonds in MoO4 tetrahedra [33, 34, 48, 49]. The experimental results for pure lead molybdate confirm the presence of only regular MoO4 tetrahedra occupying sites with the S4 symmetry in scheelite-type framework. In the case of IR spectra of the samples of solid solution, an additional absorption band, apart to those ones recorded for PbMoO4, was observed (Fig. 5). This band is clearly visible in the IR spectra of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x for x ≥ 0.0238 (at 925 cm−1 for x = 0.0238), and it moves toward higher wavenumbers up to 937 cm−1 (the saturated solid solution). Additionally, the intensity of this band significantly increases with increasing of praseodymium ions content in samples under study. Moreover, the absorption bands due to the stretching as well as bending modes of Mo–O bonds in MoO4 tetrahedra observed also in the IR spectra of PbMoO4 clearly move toward higher wave numbers when a concentration of praseodymium ions increased in doped samples. This fact suggests the presence of WO4 tetrahedra in crystal lattice of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x [48, 49]. The appearance of additional absorption band and a change in its position suggests the presence of other types of molybdenum and tungsten polyhedra, i.e., distorted MoO4 as well as WO4 tetrahedra with cationic vacancy near one or two corners of MoO4 and WO4. The same phenomenon has been observed in the case of a similar solid solution with gadolinium ions (Cd1−3x□xGd2xMoO4) [33, 34]. Gradual increase in the intensity of the additional IR band (at ~930 cm−1) and simultaneous reduction in the intensity of the bands observed at 853, 816, 768, 380, and 296 cm−1 indicate the increasing number of deformed MoO4 and WO4 tetrahedra in comparison with a number of regular ones. It seems to be evident because with increase in concentration of Pr3+ ions the number of vacancies generated in the crystal structure of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution is increased.
DTA-TG studies
Appropriate DTA-TG studies of obtained ceramic materials were preceded by similar studies for PbMoO4 and Pr2(WO4)3. Figure 6 shows DTA curves of PbMoO4 (TG curve is not presented here) recorded during heating and cooling runs. The endothermic effect started at 1040 °C is due to congruent melting of lead molybdate. The crystallization process from a melt starts at a little higher temperature, i.e., 1053 °C. These results are similar to those ones obtained by Senguttuvan et al. and by Zeng [21, 50]. Lead molybdate does not show polymorphism. Two endothermic effects were observed on the DTA curve recorded during controlled heating of Pr2(WO4)3 (Fig. 7, TG curve is not presented). The first one started at 1037 °C is connected with a phase transformation of a monoclinic α-Pr2(WO4)3 (space group C2/c) to a high-temperature polymorph (β) with a orthorhombic symmetry (space group Pnca, a structure of Sc2(MoO4)3-type) [51, 52]. A similar polymorphic change is observed in other rare-earth metal tungstates, e.g., for Gd2(WO4)3 [38]. Praseodymium tungstate melts congruently in air at 1112 °C (the second endothermic effect on DTA curve of this compound recorded during controlled heating). The crystallization process from a melt and the phase transition of β- to α-polymorph start at 1107 and 903 °C, respectively (Fig. 7). Figure 8 shows DTA curves of some samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution (Nos. 2–10). On each DTA curve only one endothermic effect (symmetrical or asymmetrical) was recorded. This effect was caused by melting of each sample under study. When a concentration of Pr3+ ions in PbMoO4 matrix increased up to x = 0.0238 (up to 2.50 mol% of praseodymium tungstate in initial PbMoO4/Pr2(WO4)3 mixtures), the onset of endothermic peak increased up to 1055 °C. The endothermic effects recorded on DTA curves of monophasic samples for x ≥ 0.0455 are clearly asymmetric with a visible inflection observed at a temperature slightly lower than a minimum temperature of each effect. This inflection can suggest a simultaneous presence of two endothermic peaks with slightly different onsets. In a case of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x samples for x ≥ 0.0455, the onset of observed peaks decreased from 1048 up to 1025 °C (for the saturated solid solution). In the whole homogeneity range melting point of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x samples shows nonlinear dependence on x parameter.
UV–Vis–NIR spectra and band gap determination
The band gap energy, E g, is a very important attribute of semiconductors because it determines their applications, e.g., in optoelectronics. Optical absorption studies are used to characterize the electronic properties of materials, through the determination of such parameters as band gap and excited state lifetime. The band gap for bulk PbMoO4 and samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution was determined from their diffuse reflectance spectra showed in Fig. 9. The reflectance data were converted into absorption ones using the Kubelka–Munk function according to the following Eq. [53]:
where R is reflectance and F(R) is the Kubelka–Munk transformation. It is known that the band gap E g and absorption coefficient α are related as in the following Eq. [53]:
where A is a constant characteristic for material under study, h is the Plank constant, ν is light frequency, and n is a constant associated with the different types of electronic transitions (n = 1/2, 2, 3/2, or 3) depending upon an nature of electronic transitions responsible for an absorption [54]. The Tauc optical gap for PbMoO4 and monophasic samples of solid solution was determined through an extrapolation of the linear trend observed in the spectral dependence of (α·hν)1/n, i.e., [F(R)·hν]1/n over a limited range of photon energies hν. The values of band gap energy for n = 2, i.e., for indirect allowed transition of an electron from a valance to a conduction band are showed in Table 1. The E g value for PbMoO4 was found to be 3.17 eV, and this value is very close to those ones reported earlier, i.e., 3.14–3.19 eV [22], 3.20 eV [23], and 3.30 eV [1]. The observed band gap of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x samples increases from 3.14 eV (x = 0.0050) up to 3.22 eV (x = 0.2000). The E g value of saturated solid solution, i.e., Pb0.3334□0.2222Pr0.4444(MoO4)0.3334(WO4)0.6666 (x = 0.2222) is a slightly lower and equals 3.21 eV. It was also shown a nonlinear (parabolic) variation in the band gap with x parameter (Fig. 10). Similar dependence of E g (nonlinear) on x parameter was observed for scheelite Ca1-x Sr x MoO4 solid solution [55].
Conclusions
Polycrystalline samples of new Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution, where □ are cationic vacancies, have been successfully synthesized by a solid-state reaction at the temperatures ranging from 900 to 975 °C. As the starting materials for synthesis, PbMoO4 and Pr2(WO4)3 were used. The homogeneity range of solid solution under study is 0 < x ≤ 0.2222, i.e., the maximum content of praseodymium tungstate in initial PbMoO4/Pr2(WO4)3 equals 40.00 mol%. All monophasic samples crystallize in a tetragonal body-centered scheelite-type structure. Samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution do not show polymorphism, and their melting point strongly depends on a value of x parameter. The highest melting point, i.e., 1055 °C is for a solid solution with x = 0.0238, and this temperature is slightly higher than a melting point of pure matrix (PbMoO4, 1040 °C). IR spectra showed a presence of MoO4 and WO4 tetrahedra in the scheelite structure of new phases. Additionally, in comparison to regular MoO4/WO4 tetrahedra occupying sites with the S4 symmetry in a scheelite-type framework, a some deformation of MoO4 and WO4 tetrahedra in structure of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x is observed. Both, pure matrix (PbMoO4) and samples of Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x solid solution are insulators with indirect band gap E g > 3 eV. The nonlinear dependence of E g on a value of x parameter is also observed.
References
Bi JH, Wu L, Zhang YF, Li ZhH, Li JQ, Fu ZhX. Solvothermal preparation, electronic structure and photocatalytic properties of PbMoO4 and SrMoO4. Appl Catal B. 2009;91:135–43.
Babin V, Bohacek P, Bender E, Krasnikov A, Mihokova E, Nikl M, Senguttuvan N, Stolovits A, Usuki Y, Zazubovich S. Decay kinetics of the green emission in tungstates and molybdates. Radiat Meas. 2004;38:533–7.
Wang J, Li X, Luo L, Zhang S, Lu R. Core–shell BaMoO4@SiO2 nanospheres: preparation, characterization, and optical properties. Ceram Int. 2013;39:9293–8.
Choi GK, Kim JR, Yoon SH, Hong KS. Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A = Mg, Zn, Mn) AMoO4 compounds. J Eur Ceram Soc. 2007;27:3063–7.
Azevedo Marques AP, de Melo DMA, Longo E, Paskocimas CA, Pizani PS, Leite ER. Photoluminescence properties of BaMoO4 amorphous thin films. J Solid State Chem. 2005;178:2346–53.
Sczancoski JC, Cavalcante LS, Joya MR, Varela JA, Pizani PS, Longo E. SrMoO4 powders processed in microwave-hydrothermal: synthesis, characterization and optical properties. Chem Eng J. 2008;140:632–7.
Kim MJ, Huh YD. Synthesis and optical properties of CaMoO4:Eu3+, Na+ nanophosphors and a transparent CaMoO4:Eu3+, Na+ suspension. Opt Mater. 2012;35:263–7.
Boulon G. Fifty years of advances in solid-state laser materials. Opt Mater. 2012;34:499–512.
Shivakumara C, Saraf R. Eu3+-activated SrMoO4 phosphors for white LEDs applications: synthesis and structural characterization. Opt Mater. 2015;42:178–86.
Hernández-Uresti DB, Martínez-de la Cruz A, Aguilar-Garib JA. Photocatalytic activity of PbMoO4 molybdate synthesized by microwave method. Catal Today. 2013;212:70–4.
Sczancoski JC, Bomio MDR, Cavalcante LS, Joya MR, Pizani PS, Ravela JA, Longo E, Siu Li M, Andrés JA. Morphology and blue photoluminescence emission of PbMoO4 processed in conventional hydrotermal. J Phys Chem C. 2009;113:5812–22.
Doroshenko ME, Basiev TT, Vassiliev SV, Ivleva LI, Komar VK, Kosmyna MB, Jelínkova H, Šulc J. Comparative study of the lasing properties of self-Raman capable Nd3+ doped tungstates and molybdates under diode pumping. Opt Mater. 2007;30:54–7.
Gyawali G, Adhikari R, Joshi B, Ho Kim T, Rodríguez-Gonzáles V, Wohn Lee S. Sonochemical synthesis of solar-light-driven Ag°-PbMoO4 photocatalyst. J Hazard Mater. 2013;263:45–51.
Aghamalyan NR, Demirkhanyan GG, Hovsepyan RK, Kostanyan RB, Zargaryan DG. Room-temperature near infrared emission and green up-conversion in PbMoO4:Er3+ crystals. Opt Mater. 2010;32:1046–9.
Xing G-J, Liu R, Zhao C, Li Y-L, Wang Y, Wu G-M. Photoluminescence and photocatalytic properties of uniform PbMoO4 polyhedral crystals synthesized by microemulsion-based solvothermal method. Ceram Int. 2011;37:2951–6.
Nedilko S, Chornii V, Hizhnyi Y, Trubitsyn M, Volnyanskaya I. Luminescence spectroscopy and electronic structure of the PbMoO4 and Pb2MoO5 single crystals. Opt Mater. 2014;36:1754–9.
Danevich FA, Grinyov BV, Henry S, Kosmyna MB, Kraus H, Krutyak N, Kudovbenko VM, Mikhailik VB, Nagornaya LL, Nazarenko BP, Nikolaiko AS, Polischuk OG, Puzikov VM, Shekhovtsov AN, Tretyak VI, Vostretsov YY. Feasibility study of PbWO4 and PbMoO4 crystal scintillators for cryogenic rare events experiments. Nucl Instr Meth Phys Res A. 2010;622:608–13.
Phuruangrat A, Thongtem T, Thongtem S. Analysis of lead molybdate and lead tungstate synthesized by a sonochemical method. Curr Appl Phys. 2010;10:342–5.
Bomio MRD, Cavalcante LS, Almeida MAP, Tranquilin RL, Batista NC, Pizani PS, Andrés J, Siu Li M, Longo E. Structural refinement, growth mechanism, infrared/Raman spectroscopies and photoluminescence properties of PbMoO4 crystals. Polyhedron. 2013;50:532–45.
Phuruangrat A, Thongtem T, Thongtem S. Synthesis of lead molybdate and lead tungstate via microwave irradiation method. J Cryst Growth. 2009;311:4076–81.
Senguttuvan N, Moorthy Babu S, Subramanian C. Synthesis, crystal growth and mechanical properties of lead molybdate. Mat Sci Eng B. 1997;47:269–73.
Gorobets YN, Kosmyna MB, Luchechko AP, Nazarenko BP, Puzikov VM, Shekhovtsov AN, Sugak DY. Crystal growth of PbWO4:Nd3+ and PbMoO4:Nd3+ crystals and their characterization by means optical and dielectric relaxation spectroscopy. J Cryst Growth. 2011;318:687–90.
Kwolek P, Tokarski T, Łokcik T, Szaciłowski K. Novel, microwave assisted route of synthesis of binary oxide semiconducting phases–PbMoO4 and PbWO4. Arch Metall Mater. 2013;58:217–22.
Chen J, Zhang Q, Liu T, Shao Z. First-principles study of color centers in PbMoO4 crystals. Phys B. 2008;403:555–8.
Guzik M, Tomaszewicz E, Kaczmarek SM, Cybińska J, Fuks H. Spectroscopic investigations of Cd0.25Gd0.50□0.25WO4:Eu3+: a new promising red phosphor. J Non-Cryst Solids. 2010;356:1902–7.
Guzik M, Tomaszewicz E, Guyot Y, Legendziewicz J, Boulon G. Structural and spectroscopic characterizations of two promising Nd-doped monoclinic or tetragonal laser tungstates. J Mater Chem. 2012;22:14896–906.
Tomaszewicz E, Kaczmarek SM, Fuks H. New cadmium and rare-earth metal tungstates with the sheelite type structure. J Rare Earths. 2009;27:569–73.
Tomaszewicz E, Fuks H, Typek J. Synthesis, thermal stability and magnetic properties of novel cadmium and praseodymium tungstate Cd0.25Pr0.50□0.25WO4 and its solid solutions. Thermochim Acta. 2013;568:95–103.
Tomaszewicz E, Filipek E, Fuks H, Typek J. Thermal and magnetic properties of new scheelite type Cd1−3x □ x Gd2x MoO4 ceramic materials. J Eur Ceram Soc. 2014;34:1511–22.
Guzik M, Tomaszewicz E, Guyot Y, Legendziewicz J, Boulon G. Structural and spectroscopic characterizations of new vacancied Cd1−3x Nd2x □ x MoO4 scheelite-type molybdates as potential optical materials. J Mater Chem C. 2015;3:4057–69.
Guzik M, Tomaszewicz E, Guyot Y, Legendziewicz J, Boulon J. Spectroscopic properties, concentration quenching and Yb3+ site occupations in a vacancied scheelite-type molybdates. J Lumin. 2016;169:755–64.
Guzik M, Tomaszewicz E, Guyot Y, Legendziewicz J, Boulon G. Eu3+ luminescence from different sites in scheelite-type cadmium molybdate red phosphor with vacancies. J Mater Chem C. 2015;3:8582–94.
Godlewska P, Tomaszewicz E, Macalik L, Hanuza J, Ptak M, Tomaszewski P, Mączka M, Ropuszyńska-Robak P. Correlation between the structural and spectroscopic parameters for Cd1−3x Gd2x □ x MoO4 solid solutions where □ denotes cationic vacancies. Mater Chem Phys. 2013;139:890–6.
Godlewska P, Tomaszewicz E, Macalik L, Hanuza J, Ptak M, Tomaszewski PE, Ropuszyńska-Robak P. Structure and vibrational properties of scheelite type Cd0.25RE0.5□0.25MoO4 solid solutions where □ is the cationic vacancy and RE = Pr, Nd, Sm–Dy. J Mol Struct. 2013;1037:332–7.
Groń T, Tomaszewicz E, Berkowski M, Duda H, Kukuła Z, Pawlus S, Mydlarz T, Ostafin T, Kusz J. Dielectric and magnetic properties of CdMoO4:Gd3+ single crystal. J Alloys Compd. 2014;593:230–4.
Macalik L, Tomaszewicz E, Ptak M, Hanuza J, Berkowski M, Mączka M, Ropuszyńska-Robak P. Polarized Raman and IR spectra of oriented Cd0.9577Gd0.0282□0.0141MoO4 and Cd0.9346Dy0.0436□0.0218MoO4 single crystals where □ denotes the cationic vacancies. Spectrochim Acta A. 2015;148:255–9.
Kukuła Z, Tomaszewicz E, Mazur S, Groń T, Pawlus S, Duda H, Mydlarz T. Electrical and magnetic properties of CdRE2W2O10 tungstates (RE = Y, Nd, Sm, Gd-Er). J Phys Chem Solids. 2013;74:86–93.
Tomaszewicz E, Fuks H, Typek J, Sawicki B, Oboz M, Groń T, Mydlarz T. Preparation, thermal stability and magnetic properties of new AgY1-x Gd x (WO4)2 ceramic materials. Ceram Int. 2015;41:5734–48.
Sawicki B, Groń T, Tomaszewicz E, Duda H, Górny K. Some optical and transport properties of a new subclass of ceramic tungstates and molybdates. Ceram Int. 2015;41:13080–9.
Šulcová P, Stránská L, Proklešková E. Study of Bi2O3 doped by rare-earth element. J Therm Anal Calorim. 2013;113:1203–8.
Öztürk E, Kalaycioglu Ozpozan N. Mn4+-, Tb3+,4+-, and Er3+-activated red phosphors in the MgAl2Si2O8 system. J Therm Anal Calorim. 2014;115:573–7.
Öztürk E, Kalaycioglu Ozpozan N, Dayan S. Novel red-emitting phosphors, (Mg(1-x-y)Mn x Dyy)Al2Si2O8 and (Mg(1-x-y)Mn x Tmy)Al2Si2O8. J Therm Anal Calorim. 2014;117:573–8.
Öztürk E, Karacaoglu E. Luminescence properties of M2TiO4:Eu3+, Li+, (M:Mg, Ca) and MgAl2O4:RE3+ (RE3+:Ho3+, Sm3+, and Yb3+). J Therm Anal Calorim. 2015;119:1063–71.
Öztürk E, Karacaoglu E. Investigation of phase formation dependency of photoluminescence properties of Eu3+ in Mg4Al2O7:Eu3+, Dy3+, and Ca4Al2O7:Eu3+, Dy3+ red-emitting phosphors. J Therm Anal Calorim. 2015;120:1139–43.
Opravil T, Ptáček P, Šoucal F, Bartoníčková E, Wasserbauer J. Solid-state synthesis of SrY2O4 and SrSm2O4. J Therm Anal Calorim. 2016;123:181–94.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;32(5):751–67.
Taupin D. A powder-diagram authomatic-indexing routine. J Appl Crystallogr. 1973;6:380–5.
Tsaryuk VI, Zolin VF. Vibration and vibronic spectra of lanthanide compounds with different types of coordination polyhedra of tungsten and molybdenum. Spectrochim Acta A. 2001;57:355–9.
Hanuza J, Mączka M, van der Maas JH. Polarized IR and Raman spectra of tetragonal NaBi(WO4)2, NaBi(MoO4)2 and LiBi(MoO4)2 single crystals with scheelite structure. J Mol Struct. 1995;348:349–52.
Zeng HC. Correlation of PbMoO4 crystal imperfections to Czochralski growth process. J Cryst Growth. 1997;171:136–45.
Brixner LH, Sleight AW. Crystal growth and precision lattice constants of some Ln2(WO4)3-type rare earth tungstates. Mat Res Bull. 1973;8:1269–74.
Nassau K, Levinstein HJ, Loiacono GM. A comprehensive study of trivalent tungstates and molybdates of the type L2(MO4)3. J Phys Chem Solids. 1965;26:1805–16.
Kubelka P, Munk F. Ein Beitrag zur Optic der Farbanstriche. Z Tech Phys. 1931;12:593–601.
Tauc J, Grigorovici R, Vancu A. Optical properties and electronic structures of amorphous germanium. Phys Status Solid. 1966;15:627–37.
Ramarao SD, Roopas Kiran S, Murthy VRK. Structural, lattice vibrational, optical and micowave dielectric studies on Ca1-x Sr x MoO4 ceramics with scheelite structure. Mat Res Bull. 2014;56:71–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Piątkowska, M., Tomaszewicz, E. Synthesis, structure, and thermal stability of new scheelite-type Pb1−3x□xPr2x(MoO4)1−3x(WO4)3x ceramic materials. J Therm Anal Calorim 126, 111–119 (2016). https://doi.org/10.1007/s10973-016-5499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5499-2