Abstract
This work is devoted to the investigation of the effect of alkali and alkaline earth metals addition on Ni/ZrO2 catalyst activity in high-temperature cellulose conversion. The catalysts containing 20 % of Ni and 1 % of Ca, Mg, Na and K (calculated per amount of oxide) introduced on ZrO2 surface by impregnation method were prepared. The surface properties of the investigated samples were characterized by X-ray diffraction, temperature-programmed reduction, flame atomic absorption spectrometry, scanning electron microscopy and energy-dispersive X-ray spectroscopy. The composition of the gaseous products was identified using gas chromatography. The performed studies demonstrated that introduction of alkali and alkaline earth metals on the surface of nickel catalyst resulted in the considerable increase in the production of hydrogen in comparison with Ni/ZrO2 reference sample. The highest hydrogen yield was formed in the presence of the catalyst modified by calcium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is considered one of the most promising renewable energy sources. Due to its high availability and relatively low price, this type of feedstock can be used for the production of alternative fuels or various high-value chemical compounds (Fig. 1) [1, 2]. The literature data demonstrate that a large number of the studies focused on the efficient formation of hydrogen-rich gas [3–5]. However, the biomass conversion is not an easy task. It results from the fact that the selectivity and productivity of this process are not satisfactory. Therefore, in order to develop a competitive method of hydrogen production from biomass, an application of the catalyst is necessary.
It was demonstrated that alkali and alkaline earth metals are catalytically active materials which can be used as independent catalysts or catalyst dopants in biomass conversion process [6, 7]. Shimada et al. [8] examined alkali/alkaline earth chlorides (NaCl, KCl, CaCl2, MgCl2) and their impact on the yield of several low molecular weight organic compounds in the cellulose pyrolysis. It was showed that the presence of alkali and alkaline earth metals allowed for a decrease in the temperature of pyrolysis. Both groups of metals had influence on the yield of low molecular weight products; however, alkaline earth metals had stronger impact on the reduction of pyrolysis temperature. The investigation performed by Acharya et al. [9] confirmed that an application of CaO as a catalytic material and its addition to biomass increased the amount of produced hydrogen in steam gasification of biomass. Rane et al. [10] described the studies focused on the effect of CaO doping with other alkali metals on its catalytic performance in oxidative coupling of methane. It was observed that introduction of other metals led to significant loss of the surface area of CaO. However, an increase in the number of basic sites, selectivity and higher hydrocarbons yield was also noticed. A combination of sodium and calcium oxides resulted in the formation of the largest amount of C2+ hydrocarbons.
On the other hand, the literature data show that due to their catalytic properties and relatively low price, nickel-based systems become the most popular catalysts in the thermo-chemical conversion of biomass [11, 12] (for example—the cost of synthesis of supported nickel catalyst using nitrate precursor is from a few to tens times lower than the price of the platinum-containing material, even taking into account introduction of lower percentage of precious metal) [13]. However, it is known that the catalytic activity and deactivation rate of Ni catalysts in the pyrolysis or gasification of lignocelluloses strongly depend on the presence and type of the applied support [12]. The results of our earlier investigations demonstrated that the use of zirconia, due to its high thermal and coking resistance, allowed to prepare the most active Ni catalyst in the cellulose conversion process [14]. However, it was shown that the presence of alkali and alkaline earth metals has also a positive effect on the performance of nickel catalyst, which is connected with their ability to adsorb CO2 formed in the high-temperature conversion of biomass and control of acid–base properties of the catalyst surface [15].
Chen et al. [16] discussed an impact of the addition of alkali and alkaline earth metals on the resistance of Ni/Al2O3 catalyst against the carbon deposit formation in catalytic cracking of n-hexane. The obtained results demonstrated that potassium was the most effective in reducing the coking rate of the investigated catalysts, while application of magnesium resulted in the smallest effect among the studied dopants. Nichele et al. [17] investigated ethanol steam reforming. They noticed that modification of Ni/ZrO2 by CaO inhibited the carbon deposition on the catalyst surface. It was reported that an addition of calcium oxide did not affect the morphology and crystalline structure of the catalyst, but it directly influenced reducibility of nickel oxide. Moreover, it was demonstrated that an increase in the CaO content resulted in a decrease in Lewis acidity of the zirconia-based catalysts which was connected with the drop in the coke deposition rate. The obtained data showed also that doping with CaO allowed for formation of oxygen vacancies which provided activated OH or O radicals the ability to stop the accumulation of carbon at the interface between Ni and the support. The investigations of carbon deposit formation on Ni/ZrO2 catalyst surface in CO2/CH4 reforming were also described by Liu et al. [18]. It was exhibited that addition of CaO resulted in the generation of basic sites on the catalyst surface which took part in the chemisorption of CO2 and promoted the gasification of deposited coke.
Wang et al. [19] studied the formation of the carbon deposit on the surface of Ni/ZrO2 catalyst modified by CaO in dry reforming of methane and linked its deactivation with the reduced accessibility of the nickel phase for the reactants. It was showed that the presence of calcium oxide allowed for the reduction of the catalyst coking rate. Moreover, it was evidenced that in CO2-rich conditions, CaCO3 rather than nickel carbide is formed on the surface of the studied sample. It confirmed chemical adsorption of carbon dioxide on CaO phase.
The research on the modification of Ni/ZrO2 by calcium conducted by Chen et al. [20] suggested the possibility of the formation of alkaline metal layer on the catalyst surface that allows the interaction between CaO or MgO and the ZrO2. The arisen extra oxygen vacancies were most likely responsible for removal of the carbon species that have already been accumulated on the catalyst. Takano et al. [21] discussed an influence of calcium oxide concentration on the support structure and catalytic performance of Ni/ZrO2 in CO2 methanation. It was exhibited that in the case of low CaO content, unfavoured monoclinic ZrO2 was formed, while in calcium-rich environment, amorphous ZrO2 or CaZrO3 phases were observed. Optimized amount of the dopant led to arising of tetragonal ZrO2 with oxygen vacancies coming from layer of alkaline metal. Introduction of calcium on tetragonal ZrO2 resulted in high catalytic activity of the prepared material.
Xu et al. [22] investigated an impact of MgO addition to Ni catalyst supported on Al2O3, TiO2 and SiO2 on its activity in CH4/CO2 reforming. The presence of solid solution of Ni–Mg–O2 was found near the surface of the catalyst. It blocked the support species and was responsible for lower activity of the modified catalyst. Higher catalytic activity was obtained for the materials doped with alkali metal. The work of Takenaka et al. [23] confirmed the possibility of the formation of solid solution and mixed phase containing nickel and magnesium oxides. Sun et al. [24] reported that an addition of MgO to the Ni/ZrO2 favoured the formation of the solid solution that prevented transformation of ZrO2 phase from tetragonal to monoclinic, which led to the substantial increase in the thermal stability of the catalyst in coal bed methane reforming to synthesis gas. An et al. [25] examined impact of both CaO and MgO on the physicochemical properties of ceria–zirconia. The significant difference was found between those two dopants. Calcium oxide was incorporated effectively into the catalyst microstructure due to proper ionic radius, which was confirmed by X-ray diffraction (XRD) measurements. In the case of MgO, small solubility of Mg2+ in ceria lattice decreased overall oxygen storage capabilities (OSC) of the catalyst and led to the collapse of its structure.
The influence of the addition of potassium and sodium oxides on activity of ZrO2–CeO2 catalyst in temperature-programmed reactions of diesel soot model compound (carbon black) was studied by Liang et al. [26]. They demonstrated that introduction of potassium was the most effective and allowed for a highest increase in activity of ceria–zirconia in the described process. The positive effect of the addition of sodium and/or potassium oxides during the preparation of zirconia was also reported by Chuah and Jaenicke [27]. They noticed that modified support was less susceptible to sintering in high temperatures and was obtained as tetragonal zirconia after preparation.
Taking into account the information presented above, we decided to investigate the influence of the addition of alkali and alkaline earth metals (Na, K, Ca and Mg) on the activity of Ni/ZrO2 catalyst in cellulose conversion process towards hydrogen rich gas. Moreover, the impact of dopants on the physicochemical properties of the synthesized materials was determined.
Experimental
Catalyst preparation
ZrO2 was prepared from ZrOCl2·8H2O (Sigma-Aldrich, pure for analysis (≥99.5 %)) by precipitation with NaOH followed by calcination at 700 °C in air. First, 200 mL of 0.4 M ZrOCl2·8H2O was added dropwise to 60 mL of 5 M NaOH (StanLab, pure for analysis). Then, the mixture was heated to 104 °C and stirred for 24 h. The precipitate was filtered on a Büchner funnel and washed with 0.05 M solution of NH4NO3 (Chempur, pure (min. 99 %)) and then with distilled water until neutral pH. It was then dried in air at 110 °C overnight and calcined in air at 700 °C for 3 h to obtain ZrO2 from Zr(OH)4 (Fig. 2). The support prepared this way was treated as a base for reference catalyst after introduction of nickel—this sample will be referred as Ni/Zr (Table 1).
In the case of the synthesis of MxOy–ZrO2 supports (where M = Ca, Mg, Na and K − calculated for the amount of oxide − 1 %), impregnation method was used. Calculated amount of alkali metal precursor: Ca(NO3)2·6H2O (Sigma-Aldrich, 99.9 %), Mg(NO3)2·6H2O (Sigma-Aldrich, 99.9 %), Na2CO3 (Chempur, 99.9 %) and K2CO3 (Chempur, 99.9 %) was dissolved in the small amount of water and added to the beaker containing a portion of ZrO2. It was stirred for several minutes and aged for 24 h at room temperature. Then, water was evaporated and supports were calcined in air flow at 700 °C for 3 h.
The supported 20 % Ni catalysts were prepared by the impregnation method. Nickel was introduced from Ni(NO3)2·6H2O (Chemland, pure for analysis (≥99.5 %)) on the ZrO2 reference and modified supports. The samples were aged for 24 h at room temperature. After evaporation of water, the catalysts were dried at 110 °C for 2 h and then calcined in air flow at 700 °C for 3 h. The prepared materials will be later referred to as 1 % M–Zr IMP (Table 1).
Catalyst characterization
The surface area of the investigated catalysts was measured by the comparative method. Low-temperature adsorption of hydrogen (gas mixture 95 vol% H2 and 5 vol% Ar with flow rate 40 mL min−1) on the sample cooled with liquid nitrogen was measured with thermal conductivity detector (TCD)—bridge current 200 mA. Al2O3 was used as a reference—surface area 123.8 m2 g−1. Before the measurements, samples were dried in crucibles at 120 °C for 2 h and then cooled down to room temperature in desiccator. About 0.1 g of the measured sample was weighed on the analytical weight, then placed in the U-tube and cooled in liquid nitrogen in a gas flow. After 5 min, liquid nitrogen thermos was switched for beaker containing water at about 5 °C and the analytical signal was collected with the usage of TCD.
Temperature-programmed reduction (TPR) was performed on AMI1 system from Altamira Instruments equipped with a TCD and used for examining the reducibility of the catalysts calcined at 700 °C. In the experiments, mixture of 5 vol% H2 and 95 vol% Ar at a flow rate of 30 mL min−1 and linear temperature ramp of 10 °C min−1 was used.
Powder XRD were collected using a PANalytical X’Pert Pro MPD diffractometer. The X-ray source was a copper long fine focus X-ray diffraction (XRD) tube operating at 40 kV and 30 mA. Data were collected in the 5°–90° 2θ range with 0.0167° step. Crystalline phases were identified by references to ICDD PDF-2 (version 2004) database. All calculations were performed with X’Pert High Score Plus computer program. Crystalline size was calculated with Scherrer method.
Scanning electron microscope (SEM) UHR FE-SEM Hitachi SU8020 and attached energy-dispersive X-ray spectroscopy (EDS) system were used for the investigation of the morphology and composition of the catalysts’ surface. The measurements were taken at the acceleration voltage of 5.0 kV and the current of about 10 μA.
Percentage of nickel deposited on the catalyst surface was examined by flame atomic absorption spectrometry (F-AAS) using GBC 932 plus instrument. The mixture of HCl (30 %, Merck) and HNO3 (65 %, Merck) in Microwave Digestion System (Anton Paar 3000) was used to extract the metal from the catalysts. After that, the solutions were transferred to 100-mL volumetric flasks. Due to the inability to fully decompose zirconia, the suspensions were filtered before the metal determination. Calibration curve for the measurements was prepared using the working standards synthesized by dissolving of commercially available nickel standard stock solution 1000 mg mL−1 (Merck).
Catalyst activity
The activity of the investigated Ni/MxOy–ZrO2 catalysts was tested in stirred batch reactor (with a volume of approximately 250 mL) under near-atmospheric pressure at 700 °C for 4 h. The reaction temperature was chosen based on preliminary measurements. The conversion of the model biomass sample—α-cellulose (Sigma-Aldrich, pure)—was conducted in the presence of nickel catalysts. In each case, 5 g of α-cellulose and 0.2 g of the catalyst were used.
An analysis of the reaction products exhibited the formation of gaseous mixture, liquid fraction and carbonaceous residue. An amount of permanent gases such as hydrogen, methane, carbon oxide and carbon dioxide was determined using gas chromatograph (GCHF 18.3, Chromosorb 102 column) equipped with a TCD. The minimal flow of Ar was used in order to direct the formed gases from the reactor to the gas chromatograph. The gaseous products were collected every 0.5 h (each time, three different gas samples were injected to gas chromatograph).
Results and discussion
The comparison of selected physicochemical properties of Ni catalysts modified by calcium, magnesium, sodium and potassium oxides with the reference Ni/ZrO2 sample is presented in Table 2. It is clear that type of the introduced dopant has a strong impact on the surface area of the investigated catalysts. The results demonstrated that addition of calcium and magnesium caused a substantial drop in the surface area of the catalysts. This effect was not observed in the case of the introduction of sodium and potassium, where the high surface area of the prepared samples was retained. Their hydroxides are used as precipitating agents in the synthesis of the high-surface zirconia, which may suggest that they have positive impact on the development of porous structure. On the other hand, decomposition of alkaline nitrates during calcination can lead to destruction of the zirconia porous structure or creation of monolayer of alkaline metal oxide that covers ZrO2 surface. Usually, such layer exhibits increased oxygen storage capacity that helps to remove carbonaceous deposit from the catalyst, which may explain why lower surface sample containing calcium reveals the highest activity in cellulose conversion process [17].
F-AAS measurements confirmed that the amount of nickel deposited on the surface of the investigated catalysts was close to 20 % which was expected. A slightly higher Ni concentration was observed for the samples containing calcium and sodium.
The results of TPR measurements are presented in Fig. 3. The TPR profiles exhibit considerable differences in the NiO reduction behaviour introduced on the surface of modified catalysts. In the case of unmodified Ni/ZrO2 sample, the reduction of the catalysts begins from 300 °C and finishes slightly below 700 °C. There is only one maximum of the hydrogen consumption rate at about 550 °C. The similar TPR run was noticed for potassium-containing sample. However, in other cases, more than one reduction peak is observed on the TPR profiles. This is especially evident for the catalysts with calcium and magnesium addition. In this case, two maxima of the hydrogen consumption rate are noticed (between 450 and 500 °C and close to 600 °C, respectively). The comparison of the area of the mentioned peaks demonstrates that for the catalyst containing magnesium, most of the hydrogen is consumed below 500 °C, while in the case of the catalyst with the addition of calcium, the areas of both peaks are similar. The obtained results might suggest the presence of the interaction between active phase and support, of which strength can change for different samples or creation of two types of crystallites on the sample surface after introduction of dopants. Further case can be linked with the existence of both a fraction of bigger slowly reduced crystallites and smaller NiO particles that undergo reduction in gas mixture very quickly. The similar phenomenon was reported by Youn et al. [28].
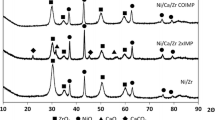
The next step of the studies was devoted to XRD measurements. In all cases, the X-ray diffractograms revealed the presence of diffraction lines at 2θ values of 37.2; 43.1, 62.8; 75.3, 79.4 and 29.8; 34.8; 49.5, 59.4 originating from NiO and ZrO2 tetragonal phases, respectively (Fig. 4). The diffraction lines corresponding to the presence of alkali or alkaline earth metal oxides were not observed (probably due to the formation of amorphous layer on the support surface). The XRD method was also used for the estimation of NiO crystallite size (Table 2). It was calculated with the use of Scherrer equation [29]. The largest nickel oxide crystallites were noticed for the samples doped with calcium and magnesium (36 and 31 nm, respectively). The samples with the addition of sodium and potassium contained smaller NiO particles (about 21–23 nm) with the size similar to that observed for unmodified Ni/ZrO2 catalyst (26 nm).
SEM–EDS measurements were taken in order to compare the composition and morphology of the surface of the modified catalysts. The obtained results (Table 3) showed that despite low surface area, the highest amount of nickel was present on the surface of calcium-doped sample (33.5 %). In the case of the catalyst modified with magnesium, the Ni content was lower (25.6 %) but still noticeably higher than in the case of the samples containing sodium and potassium. The reverse regularity was observed for zirconium. The investigation of the concentration of alkali and alkaline earth metals showed that sodium was the most abundant among the studied elements. However, it should be taken into consideration that NaOH was used as precipitating agent in the synthesis of ZrO2 support. Therefore, the amount of this metal is the sum of atoms introduced in the impregnation of the catalyst with sodium carbonate and synthesis of ZrO2 with the use of sodium hydroxide.
The SEM images collected from the surface of the modified catalysts are presented in Fig. 5. They demonstrated that alkali and alkaline earth metal oxides cover the surface of Ni/ZrO2 catalyst. The particle size of the oxides varied depending on the kind of the used metal. The larger grains were observed in the case of calcium oxide (about 0.2–0.5 μm) and magnesium oxide (in the range of 0.5–1 μm), while for sodium oxide and potassium oxide, the particles of smaller size were found (0.1–0.2 μm).
The catalytic activity of the modified catalysts was investigated in the high-temperature cellulose conversion process. Moreover, the experiments with reference Ni/ZrO2 sample were performed for comparison. The activity tests revealed that besides gaseous fraction, also liquid phase and solid residue (carbonaceous material) were formed. It was noticed that amount of liquid fraction was doubled when magnesium and potassium were added to the catalyst (~0.6–0.7 g), as compared to samples containing calcium and sodium (~0.3–0.4 g). The composition of liquid phase was very complex. It contained a mixture of different organic compounds such as hydrocarbons, carboxylic acids, aldehydes, ketones, alcohols.
The results of activity tests revealed that the main gaseous products of the studied process were hydrogen, carbon dioxide, carbon oxide and methane. However, the amount of the produced H2 and CO2 was noticeably higher than the produced CO and especially than CH4 yield.
A comparison of the catalytic performance of the tested catalysts exhibited that application of the modified samples allowed for the formation of considerably higher amount of hydrogen (13–17 mmol g−1 cellulose) than it was observed for reference Ni/ZrO2 (slightly more than 10 mmol g−1 cellulose) (Fig. 6). The production of CO2 varied between 10 mmol g−1 cellulose and 13 mmol g−1 cellulose for all analysed catalysts, and its amount slightly exceeded the yield of hydrogen only in the case of unmodified Ni/ZrO2. As it was mentioned, the production of CO and CH4 was lower and ranged from 6 to 9 and from 1 to 2 mmol g−1, respectively. It was observed that addition of alkali and alkaline earth metals resulted in more efficient formation of hydrogen, while the amount of other gaseous products did not change as much as H2. It caused the increase in the value of H2/CO and H2/CO2 ratio in the gaseous phase (from 1.33 and 0.94 for unmodified sample to 1.76–2.48 and 1.16–1.30 in the case of modified catalysts, respectively) (Table 4). Cellulose pyrolysis process is very complex, and there are many simultaneous reactions taking place including formation of hydrogen, carbon dioxide and monoxide, water, volatile organic compounds (VOC), liquid fraction containing heavier compounds (tar) and carbonaceous residue (char) (1). The obtained results suggest that modified catalysts can adsorb carbon dioxide on their surface, which shifts the equilibrium of the water gas shift reaction to the right and allows for the increase in the amount of the formed hydrogen (2) [3].
On the other hand, an increase in the value of H2/CO ratio in the presence of the doped catalysts may indicate that in this case, decomposition of cellulose occurs more readily via decarboxylation than via decarbonylation route comparing to unmodified material [30].
Mechanism of the interaction between gas phase, nickel grains and support surface was proposed by Sun et al. [31]. According to their studies, the residual carbon can be removed from the catalyst surface in the subsequent CO2 pulse by reverse Boudouard reaction. This way, the nickel phase remains free from the carbon deposit and ready for the next catalytic act to occur.
The activity tests revealed also that the addition of calcium and magnesium to Ni/ZrO2 catalyst resulted in an increase in the hydrogen production even before reaching the set temperature of the process, while the samples containing sodium and potassium were more active at the highest reaction temperature −700 °C.
The literature data [32] suggest that the deactivation rate of the catalyst in the investigated process is associated with the formation of carbon deposit. It seems that alkali and alkaline earth dopants inhibited this phenomenon, which in turn enhances overall activity of the modified materials [17, 25]. The limitation of the carbon deposit formation allows also to maintain catalytic activity of the tested systems for a longer time [31]. Unfortunately, in our case, the amount of deposited coke could not be determined due to the fact that the catalyst was mixed with the solid residue in the reactor during the reaction.
Conclusions
The effect of the addition of alkali and alkaline earth metals (Na, K, Ca and Mg) on the activity of Ni/ZrO2 catalyst in cellulose conversion process towards hydrogen rich gas was investigated. The obtained results revealed that introduction of dopants on the surface of nickel catalyst led to the considerable increase in the production of hydrogen in comparison with unmodified sample. The highest hydrogen yield was observed in the presence of the sample containing calcium. SEM–EDS measurements demonstrated that this catalyst retained the highest amount of nickel on its surface after the preparation step. It is suggested that NiO particles are not so easily deactivated with carbon deposition in the presence of used dopants, which is beneficial for the enhanced catalytic activity and increase in the hydrogen yield. Furthermore, adsorption of carbon dioxide on the surface of the modified catalysts can additionally shift the equilibrium of the occurred reactions and allow to form higher amount of H2. However, further measurements are necessary to explain the mechanism of the studied process in detail. The next step of the investigation should be also focused on the optimization of the catalyst synthesis method and content of dopants.
References
Oliveira TJP, Cardoso CR, Ataide CH. Fast pyrolysis of soybean hulls: analysis of bio-oil produced in a fluidized bed reactor and of vapor obtained in analytical pyrolysis. J Therm Anal Calorim. 2015;120:427–38.
Huang L, Ding T, Liu R, Cai J. Prediction of concentration profiles and theoretical yields in lignocellulosic biomass pyrolysis. J Therm Anal Calorim. 2015;120:1473–82.
Bulushev DA, Ross JRH. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: a review. Catal Today. 2011;171(1):1–13.
Minowa T, Ogi T. Hydrogen production from cellulose using a reduced nickel catalyst. Catal Today. 1998;45:411–6.
Zhang H, Xiao R, Song M, Shen D, Liu J. Hydrogen production from bio-oil by chemical looping reforming. Characteristics of the synthesized metal organic frameworks for CO2 removal. J Therm Anal Calorim. 2014;115:1921–7.
Li R, Kai X, Yang T, Sun Y, He Y, Shen S. Release and transformation of alkali metals during co-combustion of coal and sulfur-rich wheat straw. Energy Convers Manag. 2014;83:197–202.
Wei X, Schnell U, Hein K. Behaviour of gaseous chlorine and alkali metals during biomass thermal utilisation. Fuel. 2005;84(7–8):841–8.
Shimada N, Kawamoto H, Saka S. Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J Anal and Appl Pyrol. 2008;81(1):80–7.
Acharya B, Dutta A, Basu P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int J Hydrogen Energy. 2010;35(4):1582–9.
Rane VH, Chaudhari ST, Choudhary VR. Influence of alkali metal doping on surface properties and catalytic activity/selectivity of CaO catalysts in oxidative coupling of methane. J Nat Gas Chem. 2008;17:313–20.
Wu C, Wang Z, Dupont V, Huang J, Williams PT. Nickel-catalysed pyrolysis/gasification of biomass components. J Anal Appl Pyrol. 2013;99:143–8.
Sutton D, Kelleher B, Ross JRH. Review of literature on catalysts for biomass gasification. Fuel Process Technol. 2001;73:155–73.
http://www.sigmaaldrich.com/poland.html. Accessed 14 Mar 2016.
Ruppert AM, Niewiadomski M, Grams J, Kwapiński W. Optimization of Ni/ZrO2 catalytic performance in thermochemical cellulose conversion for enhanced hydrogen production. Appl Catal B-Environ. 2014;145:85–90.
Masnadi MS, Grace JR, Bi XT, Lim CJ, Ellis N. From fossil fuels towards renewables: inhibitory and catalytic effects on carbon thermochemical conversion during co-gasification of biomass with fossil fuels. Appl Energy. 2015;140:196–209.
Chen I, Chen F-L. Effect of alkali and alkaline-earth metals on the resistivity to coke formation and sintering of nickel–alumina catalysts. Ind Eng Chem Res. 1990;29(4):534–9.
Nichele V, Signoretto M, Pinna F, Menegazzo F, Rossetti I, Cruciani G, et al. Ni/ZrO2 catalysts in ethanol steam reforming: inhibition of coke formation by CaO-doping. Appl Catal B-Environ. 2014;150–151:12–20.
Liu S, Guan L, Li J, Zhao N, Wei W, Sun Y. CO2 reforming of CH4 over stabilized mesoporous Ni–CaO–ZrO2 composites. Fuel. 2008;87(12):2477–81.
Wang C, Sun N, Zhao N, Wei W, Sun Y, Sun C, et al. Coking and deactivation of a mesoporous Ni–CaO–ZrO2 catalyst in dry reforming of methane: a study under different feeding compositions. Fuel. 2015;143:527–35.
Chen QJ, Zhang J, Jin QW, Pan BR, Kong WB, Zhao TJ, et al. Effect of reflux digestion treatment on the catalytic performance of Ni–CaO–ZrO2 nanocomposite catalysts for CO2 reforming of CH4. Catal Today. 2013;215:251–9.
Takano H, Shinomiya H, Izumiya K, Kumagai N, Habazaki H, Hashimoto K. CO2 methanation of Ni catalysts supported on tetragonal ZrO2 doped with Ca2+ and Ni2+ ions. Int J Hydrogen Energy. 2015;40(26):8347–55.
Xu Z, Li Y, Zhang J, Chang L, Zhou R, Duan Z. Bound-state Ni species—a superior form in Ni-based catalyst for CH4/CO2 reforming. Appl Catal A-Gen. 2001;210:45–53.
Takenaka S, Ogihara H, Yamanaka I, Otsuka K. Decomposition of methane over supported-Ni catalysts: effects of the supports on the catalytic lifetime. Appl Catal A-Gen. 2001;217:101–10.
Sun L, Tan Y, Zhang Q, Xie H, Han Y. Combined air partial oxidation and CO2 reforming of coal bed methane to synthesis gas over co-precipitated Ni–Mg–ZrO2 catalyst. Int J Hydrogen Energy. 2011;36(19):12259–67.
An Y, Shen M, Wang J. Comparison of the microstructure and oxygen storage capacity modification of Ce0.67Zr0.33O2 from CaO and MgO doping. J Alloys Compd. 2007;441(1–2):305–10.
Liang H, Wu S, Hong Y, Li S, Chen Y, Yu X, et al. Influence of alkali metals with different ionic radius doping into Ce0.7Zr0.3O2 on the active oxygen. Catal Lett. 2014;144:685–90.
Chuah GK, Jaenicke S. The preparation of high surface area zirconia—influence of precipitating agent and digestion. Appl Catal A-Gen. 1997;163:261–73.
Youn MH, Seo JG, Song IK. Hydrogen production by auto-thermal reforming of ethanol over nickel catalyst supported on metal oxide-stabilized zirconia. Int J Hydrogen Energy. 2010;35(8):3490–8.
Scherrer P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Ges d Wiss Nachrichten Math-Phys Klasse. 1918;2:98–100.
Wang K, Kim KH, Brown RC. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014;16(2):727–35.
Sun N, Wen X, Wang F, Peng W, Zhao N, Xiao F, et al. Catalytic performance and characterization of Ni–CaO–ZrO2 catalysts for dry reforming of methane. Appl Surf Sci. 2011;257(21):9169–76.
Nasir Uddin M, Daud WMAW, Abbas HF. Potential hydrogen and non-condensable gases production from biomass pyrolysis: insights into the process variables. Renew Sustain Energy Rev. 2013;27:204–24.
Acknowledgements
The authors gratefully acknowledge that this work was financially supported by the National Science Center (Poland)—project 2011/03/B/ST5/03270. J. G. acknowledges the support of the President of Lodz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ryczkowski, R., Niewiadomski, M., Michalkiewicz, B. et al. Effect of alkali and alkaline earth metals addition on Ni/ZrO2 catalyst activity in cellulose conversion. J Therm Anal Calorim 126, 103–110 (2016). https://doi.org/10.1007/s10973-016-5465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5465-z