Abstract
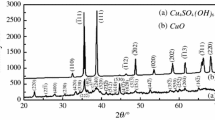
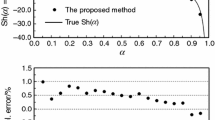
Overlapping of parallel or consecutive reactions caused to variation of activation energy and pre-exponential factor and deconvolution of simultaneous reactions rather than fitting the experimental curve by assuming variable kinetic parameters could supply a deeper conception into the mechanism(s) of reaction. The well-known reaction model determination methods are based on the choice of constant Arrhenius parameters and the use of approximations. To solve this limitation, an advanced method of reaction mechanism model determination based on the Arrhenius parameters variation was proposed. This method appears to accurately simulate single step as well as multi-step reactions kinetics. The proposed method was experimentally verified by taking an experimental example of non-isothermal desulfurization kinetics of CuO·CuSO4 in the nanoscale range. Then, the physical meaning of models was effectively interpreted.







Similar content being viewed by others
References
Tomashevitch KV, Kalinin SV, Vertegel AA, Oleinikov NN, Ketsko VA, Tretyakov YD. Application of non-linear heating regime for the determination of activation energy and kinetic parameters of solid-state reactions. Thermochim Acta. 1998;323:101–7.
Maciejewski M. Computational aspects of kinetic analysis; Part B: The ICTAC Kinetics Project—the decomposition kinetics of calcium carbonate revisited, or some tips on survival in the kinetic minefield. Thermochim Acta. 2000;355:145–54.
Vyazovkin S. Computational aspects of kinetic analysis; Part C: The ICTAC Kinetics Project—the light at the end of the tunnel. Thermochim Acta. 2000;355:155–63.
Burnham AK. Computational aspects of kinetic analysis; Part D: The ICTAC Kinetics Project—multi-thermal-history model-fitting methods and their relation to isoconversional methods. Thermochim Acta. 2000;355:165–70.
Roduit B. Computational aspects of kinetic analysis; Part E: The ICTAC Kinetics Project—numerical techniques and kinetics of solid state processes. Thermochim Acta. 2000;355:171–80.
Badea M, Budrugeac P, Cucos A, Segal E. Thermal decomposition kinetics of bis(pyridine)manganese(II) chloride. J Therm Anal Calorim. 2014;115:1999–2005.
Muraleedharan K. Thermal decomposition kinetics of potassium iodate. Part I. J Therm Anal Calorim. 2011;103:943–55.
Chaiyo N, Muanghlua R, Niemcharoen S, Boonchom B, Seeharaj P, Vittayakorn N. Non-isothermal kinetics of the thermal decomposition of sodium oxalate Na2C2O4. J Therm Anal Calorim. 2012;107:1023–9.
Ak M, Cilgi GK, Kuru FD, Cetisli H. Thermal decomposition kinetics of polypyrrole and its star shaped copolymer. J Therm Anal Calorim. 2013;111:1627–32.
Muraleedharan K. Thermal decomposition kinetics of potassium iodate. Part II. J Therm Anal Calorim. 2013;114:491–6.
Yilmaz MS, Kalpakli Y, Piskin S. Thermal behavior and dehydroxylation kinetics of naturally occurring sepiolite and bentonite. J Therm Anal Calorim. 2013;114:1191–9.
Chen WT, Chen WC, Hsueh KH, Chiu CW, Shu CM. Thermokinetic parameters analysis for 1,1-bis-(tert-butylperoxy)-3,3,5-trimethylcyclohexane at isothermal conditions for safety assessment. J Therm Anal Calorim. 2014;118:1085–94.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Simon P, Thomas P, Dubaj T, Cibulkova Z, Pellar A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in the case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9.
Burnham AK, Dinh LN. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J Therm Anal Calorim. 2007;89:479–90.
Dueramae I, Jubsilp C, Takeichi T, Rimdusit S. Thermal degradation mechanism of highly filled nano-SiO2 and polybenzoxazine. J Therm Anal Calorim. 2014;116:435–46.
Arshad MA, Maaroufi AK. An innovative reaction model determination methodology in solid state kinetics based on variable activation energy. Thermochim Acta. 2014;585:25–35.
Carr RW. Modeling of chemical reactions. 1st ed. Amsterdam: Elsevier Science; 2007.
Wan J, Bu ZY, Xu CJ, Li BG, Fan H. Preparation, curing kinetics, and properties of a novel low-volatile star-like aliphatic-polyamine curing agent for epoxy resins. Chem Eng J. 2011;171:357–67.
Santiago D, Francos XF, Ramis X, Salla JM, Sangermano M. Comparative curing kinetics and thermal–mechanical properties of DGEBA thermosets cured with a hyperbranched poly (ethyleneimine) and an aliphatic triamine. Thermochim Acta. 2011;526:9–21.
Koga N, Criado JM, Tanaka H. Reaction pathway and kinetics of the thermal decomposition of synthetic brochantite. J Therm Anal. 1997;49:1467–75.
Shahcheraghi SH, Khayati GR, Ranjbar M. An advanced reaction model determination methodology in solid-state kinetics based on Arrhenius parameters variation—Part I: Thermal dehydration kinetic analysis of Cu4SO4(OH)6. J Therm Anal Calorim. 2015. doi:10.1007/s10973-015-4708-8.
Shahcheraghi SH, Khayati GR, Ranjbar M. An advanced reaction model determination methodology in solid-state kinetics based on Arrhenius parameters variation—Part II: Thermal crystallization kinetic analysis of amorphous Cu4SO4O3. J Therm Anal Calorim. 2015 (under review).
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Liu XW, Feng YL, Li HR, Zhang P, Wang P. Thermal decomposition kinetics of magnesite from thermogravimetric data. J Therm Anal Calorim. 2011;107:407–12.
Verma UN, Mukhopadhyay K. Solid state kinetics of Cu(II) complex of [2-(1,2,3,4-thiatriazole-5-yliminomethyl)-phenol] from thermo gravimetric analysis. J Therm Anal Calorim. 2011;104:1071–5.
Selvakumar J, Raghunathan VS, Nagaraja KS. Sublimation kinetics of scandium β-diketonates. J Therm Anal Calorim. 2010;100:155–61.
Shahcheraghi SH, Khayati GR. Arrhenius parameters determination in non-isothermal conditions for mechanically activated Ag2O–graphite mixture. J Trans Nonferrous Met Soc China. 2014;24:3994–4003.
Shahcheraghi SH, Khayati GR. Kinetics analysis of the non-isothermal decomposition of Ag2O–graphite mixture. J Trans Nonferrous Met Soc China. 2014;24:2991–3000.
Shahcheraghi SH, Khayati GR. The effect of mechanical activation on non-isothermal decomposition kinetics of Ag2O–graphite mixture. The Arab J Sci Eng. 2014;39:7503–12.
Vyazovkin S. Model-free kinetics: staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Chrissafis K. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and non-isothermal data. Thermochim Acta. 1999;340–341:53–68.
Vyazovkin S. Thermal analysis. Anal Chem. 2010;82:4936–49.
Sbirrazzuoli N. Is the Friedman method applicable to transformations with temperature dependent reaction heat? Macromol Chem Phys. 2007;208:1592–7.
Vyazovkin S, Sbirrazzuoli N. Kinetic analysis of isothermal cures performed below the limiting glass transition temperature. Macromol Rapid Commun. 2000;21:85–90.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4·3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98:863–71.
Habashi F. Recent trends in extractive metallurgy. J Min Metall Sect B Metall. 2009;45:1–13.
Gaskell DR. Introduction to metallurgical thermodynamics. 4th ed. London: Taylor & Francis Books; 2003.
Jankovic B, Mentus S, Jelic D. A kinetic study of non-isothermal decomposition process of anhydrous nickel nitrate under air atmosphere. Phys B. 2009;404:2263–9.
Boonchom B. Kinetics and thermodynamic properties of the thermal decomposition of manganese di hydrogen phosphate dehydrate. J Chem Eng Data. 2008;53:1533–8.
Gao X, Dollimore D. The thermal decomposition of oxalates: Part 26. A kinetic study of the thermal decomposition of manganese(II) oxalate dehydrate. Thermochim Acta. 1993;215:47–63.
Vlaev LT, Nikolova MM, Gospodinov GG. Non-isothermal kinetics of dehydration of some selenite hexahydrates. J Solid State Chem. 2004;177:2663–9.
Jackson KA. Kinetic processes: crystal growth, diffusion, and phase transitions in materials. 1st ed. London: Wiley-VCH; 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahcheraghi, S.H., Khayati, G.R. & Ranjbar, M. An advanced reaction model determination methodology in solid-state kinetics based on Arrhenius parameters variation. J Therm Anal Calorim 123, 221–229 (2016). https://doi.org/10.1007/s10973-015-4853-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4853-0