Abstract
The phase equilibria occurring in the YPO4–Rb3PO4 system were investigated by thermoanalytical methods, X-ray powder diffraction, and ICP-OES. On the basis of the obtained results, its phase diagram is proposed. It was found that the system includes two intermediate compounds Rb3Y(PO4)2 and Rb3Y2(PO4)3. The Rb3Y(PO4)2 compound melts congruently at 1300 °C. The Rb3Y2(PO4)3 orthophosphate was previously unknown. This intermediate compound is high-temperature unstable and decomposes within the temperature range 1300–1330 °C to YPO4 and Rb3Y(PO4)2. The decomposition process is irreversible. It was found that the Rb3Y2(PO4)3 orthophosphate is isostructural with Rb3Yb2(PO4)3 and crystallizes in the cubic system (a = 1.70226 nm).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much study has been published about the binary orthophosphates M I3 Ln(PO4)2 and M I3 Ln2(PO4)3 (where MI = alkali metal or other monovalent metals, and Ln = a rare-earth element or Y or Sc) as well as those doped with lanthanide ions (see, e.g., [1–15]). Information in the literature are mainly focused on the preparation methods, crystalline structure and application possibilities of those compounds. According to the data, these materials are of technological importance for applications in optics and electronics.
It results from experience, both of other workers’ and ours, that the binary orthophosphates mentioned above may occur as intermediate compounds in the systems of the type LnPO4–M I3 PO4 [6, 16–22]. Accordingly, in view of utilitarian character of those binary compounds, we undertook research on rubidium–yttrium orthophosphates, i.e., the YPO4–Rb3PO4 system whose phase diagram was not published previously. We were also hoping to contribute to the knowledge about the chemical stability of the binary rubidium and yttrium phosphates.
From the literature information, it is known that Rb3Y(PO4)2 exists. According to [3], it melts at ~1255 °C. Its crystal structure is closely related to that of hexagonal glaserite (after [7]). Lattice parameters of Rb3Y(PO4)2 are: a = 0.5695, c = 0.8122 nm (after [3]); a = 0.566754(3), c = 0.807782(4) nm (after [7]). It was shown in [3] that heating the phosphates Rb3Ln(PO4)2 (where Ln = Gd–Lu, Y, and Sc) above of 1250 °C results in their decomposition, which is accompanied by a mass loss due to an evaporation of rubidium orthophosphate; the decomposition process proceeds according to the scheme:
We have not succeeded in finding any literature data about the existence of an expected Rb3Y2(PO4)3 binary rubidium and yttrium orthophosphate. However, the Na3Y2(PO4)3 binary sodium and yttrium orthophosphate is known [16]. This melts congruently at a temperature of 1635 °C, is stable down to room temperature and appears in two polymorphic modifications with the transition point 470 °C. Also, another orthophosphate, Rb3Yb2(PO4)3, is known [10], which crystallizes in the regular system (s.g. I21/3, a = 1.6870(10) nm, Z = 16). It melts incongruently at 1450 K and decomposes in the temperature range from 1450 to 1541 K according to the reaction scheme:
The parent orthophosphates YPO4 and Rb3PO4 are known to congruently melt at 2150 [24] and 1450 °C [25], respectively. YPO4 crystallizes in the tetragonal system (s.g. I41/amd; a = 0.68840(3), c = 0.60202(3) nm) [23]. Rb3PO4 appears in two polymorphic modifications: the low temperature one crystallizes in the orthorhombic system (s.g. Pnma, a = 1.17362(2), b = 0.81046(1), c = 0.615167(9) nm [26]), and the high-temperature one does in the regular system (s.g. Fm3, a = 0.844 nm [27]). The Rb3PO4 is strongly hygroscopic; DTA/TG–heating curves indicated a mass loss (water losing) in the temperature range 100–430 °C. A slow loss of mass was observed, too, starting from about 1150 °C, which did not affect the stoichiometry of the orthophosphate [25].
In this article, the results of investigation of the YPO4–Rb3PO4 system are described and its phase diagram is proposed.
Experimental
Investigations of the phase equilibria in the YPO4–Rb3PO4 system were conducted using the thermoanalytical methods, X-ray powder diffraction and inductively coupled plasma optical emission spectroscopy (ICP-OES). The following commercial materials (all analytically pure) were used: Y2O3 (99.92%), H3PO4 (85%), Rb2CO3, NH4H2PO4, (NH4)2HPO4. Orthophosphates YPO4 and Rb3PO4 were self-prepared.
Yttrium orthophosphate YPO4 was obtained from the following solution: 0.4 wt% Y2O3, 15 wt% P2O5 (as H3PO4), 84.6 wt% distilled water—as we reported earlier [24]. Rubidium orthophosphate Rb3PO4 was produced from a stoichiometric mixture of dry Rb2CO3 (dried at 250 °C) and (NH4)2HPO4 [25].
Samples for investigations of the YPO4–Rb3PO4 system were synthesized by a solid state reaction. The synthesis was preceded by the following mechanical processing. The weight amounts (in the assumed stoichiometric ratios) of the substrates were shaken in a weighing bottle, rubbed in an agate mortar in acetone, dried at 120 °C, placed in platinum crucibles or encapsulated in platinum ampoules, and then sintered. The synthesis conditions (i.e., temperature and time of sintering) were determined experimentally. The obtained sinters were crushed and finely ground.
The thermoanalytical investigations were carried out using a SETSYS™ (SETARAM) differential thermal analyzer (calorimeter) with balance. The device enables one to perform simultaneous TGA–DTA or TGA–DSC measurements in the temperature range from 20 to 1350 °C. Samples of weight 15–20 mg were placed in platinum crucibles and heated at a rate of 10°Cmin−1 in an atmosphere of argon. DTA and TG analyses were also carried out in the air, up to 1400 °C, by using a derivatograph type 3427 (MOM, Hungary) with a heating rate of 5 °C min−1, in platinum crucibles, and samples from 0.3 to 0.6 g. High-purity Al2O3 was used as the standard material. Temperature was measured by means of a Pt/PtRh10 thermocouple which was calibrated against the melting points of NaCl, K2SO4, and Ca2P2O7 and the transition point of K2SO4.
The high-temperature experiments (above 1400 °C) were performed in a horizontal resistance furnace with molybdenum winding, under argon. Test samples, each weighing ca. 2 g, were pelletized and placed in a Pt/PtRh30 boat. The temperature, at which a sample was diffusing and got blurred in the field of observation to finally disappear, was read by means of an optical pyrometer. For the samples that melt in a range of temperature, the melting points determined are approximate. The optical pyrometer was calibrated against the melting points of Na3PO4 and Ca3(PO4)2. The quenching-in-ice technique was also employed to determine the phases; a high-temperature vertical tubular furnace (20–1750 °C; Nabertherm RHTV 120–300/18) was used.
The phase purity of the self-prepared parent phosphates and the phase composition of both the sinters (i.e., synthesized by the solid state reaction) and the melted samples for the investigated system, were controlled by X-ray powder diffraction at room temperature. A SIEMENS D 5000 diffractometer were used. The ICDD PDF-4+ database and the structures reported in the literature were searched to detect the phases present in the samples. The quantitative analysis was performed using the ICP-OES techniques (spectrometer Vista-MPX, VARIAN, Australia).
Results and discussion
The YPO4–Rb3PO4 system was examined in the whole composition range and in up to 1800 °C. At the initial stage, non-equimolar mixtures of the orthophosphates YPO4 and Rb3PO4 (and also YPO4, Rb2CO3, and NH4H2PO4) were examined by different thermoanalytical methods. During X-ray diffraction analysis of reaction products, it was observed that some XRD patterns showed reflections indicating the appearance of certain new phases. During investigating the phase equilibria, both in binary and multicomponent systems, it is of primary importance to have possible intermediate phases identified. At the next stage, the attention was paid to intermediate compounds in the YPO4–Rb3PO4 system.
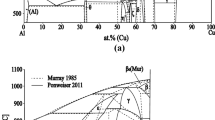
According to the literature data [3, 7], a molar ratio of YPO4:Rb3PO4 = 1:1 (34.4 wt% YPO4, 65.6 wt% Rb3PO4) corresponds to the Rb3Y(PO4)2 binary rubidium and yttrium orthophosphate. The other expected, unknown compound Rb3Y2(PO4)3 should have a molar ratio of YPO4:Rb3PO4 = 2:1 (51.14 wt% YPO4 and 48.86 wt% Rb3PO4).
In order to obtain the Rb3Y(PO4)2 orthophosphate the procedure was as follows. The parent substances: (1) YPO4, Rb2CO3, and NH4H2PO4 were mixed in the molar ratio 2:3:2, and—after mechanical processing (see “Experimental” section)—were sintered at 250 °C for 5 h, continued at 950 °C for 72 h (applying intermediate grindings to insure a total reaction); (2) YPO4 and Rb3PO4 were mixed in the molar ratio 1:1 and, after mechanical processing, sintered at 950 °C for 4 h.
The synthesis conditions of (1) and (2) were found from experimental tests. The phase composition of the sinters obtained via the above solid state processes was checked by X-ray monitoring. The product of synthesis (1) as well as (2), in the form of white powder, was phase purity Rb3Y(PO4)2. According to [3], this phosphate melts at 1255 °C. Based on the present investigation using DTA-heating in the range from room temperature to 1350 °C we have found that Rb3Y(PO4)2 melts congruently at 1304 °C. Simultaneously, a slow gradual loss of mass about 3.2 wt% was observed on the TG-heating curve from a temperature of ~1225 °C. The XRD pattern, however, of the sample slowly cooled down from ~1304 °C to room temperature showed only reflections typical of Rb3Y(PO4)2.
Consequently, a quantitative determination of rubidium, yttrium and phosphorus content was made for the samples:
-
Rb3Y(PO4)2 as synthesized in the solid state (a sinter),
-
Rb3Y(PO4)2 as melted at 1304 °C and cooled down to room temperature.
Quantitative analysis was performed using the ICP-OES method. It appeared that the content of yttrium, rubidium, and phosphorus was the same in both samples under analysis.
According to [3], Rb3Ln(PO4)2 are high-temperature unstable and upon a long heating above 1250 °C decompose to Rb3PO4 i LnPO4. In contrast, we have observed that the decomposition process has a rather complex character. It results from X-ray diffractometry that Rb3Y(PO4)2 remains its stoichiometry both upon melting (1304 °C) and upon a short (15 min) heating at 1330 and 1350 °C. In each case a mass loss was about 0.8 wt%, but XRD patterns exhibited only Rb3Y(PO4)2 reflections. Consequently, the investigation was continued by heating at 1400 °C for 1 h, which gave a mass loss of about 3.1 wt%. Once more any decomposition products expected after [3] were not found in the XRD pattern.
The scheme of thermal dissociation Rb3Y(PO4)2 given in [3], i.e.,
is a simplified form. According to [25], at starting temperature 1150 °C, Rb3PO4 slowly decomposes into rubidium oxide (gas phase) and phosphorus oxide (gas phase). What is more, the results [28, 29] indicate that lanthanide orthophosphates undergo thermal dissociation in the air above 1200 °C. The decomposition proceeds according to the scheme:

(where LnxPyOz represents four oxyphosphate groups). According to, [28] YPO4 decomposes above 1300 °C in the solid state.
The above discussed results in more detail elucidate the effect of the mass loss appearance during heating the Rb3Y(PO4)2 at high temperatures. The decomposition product YPO4 (or yttrium oxyphosphates) are not visible in the XRD pattern due to their insignificant amounts of ~1 wt% (for the total mass loss 3.1 wt%). We have confirmed in the above mentioned investigation that, in accord with the results of [3, 7], Rb3Y(PO4)2 occurs in the temperature range interest as one polymorphic modification of the hexagonal structure, and its parameters are a = 0.566095, c = 0.807379 nm.
To verify the question about the existence of the Rb3Y2(PO4)3 an attempt to synthesize it was made from the following parent substances.
-
1
YPO4, Rb3PO4
-
2
YPO4, Rb3Y(PO4)2
-
3
YPO4, Rb2CO3, and NH4H2PO4.
After mechanical processing (see “Experimental” section), the above mixtures were pressed into pellets and placed in platinum crucibles or encapsulated in platinum ampoules to be sintered at different temperatures for different periods of time. The phase composition of the output sinters was identified by X-ray powder analysis at room temperature.
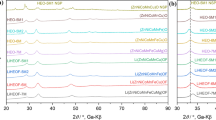
It was found that via solid state reaction of sintering an equimolar mixture of YPO4 and Rb3Y(PO4)2 in the closed ampoules at 1200 °C for 3 h, a new compound of phase purity appears. The formula Rb3Y2(PO4)3 was assigned to it. Phase transformation of Rb3Y2(PO4)3 from room temperature to 1350 °C were investigated using DTA-heating (in the air). The results are shown in Fig. 1. A slower pace is clearly seen from the temperature curve (T) in the range 1300–1330 °C. The DTA curve revealed a strong and broad thermal effect. In turn, TGA curve showed a small change in slope (a laminar lowering) above the temperature 1210 °C, which indicated a slow gradual mass loss. This loss amounted to 2.8 wt% in the temperature range 1210–1350 °C.
To explain the high-temperature behavior of the Rb3Y2(PO4)3 orthophosphate X-ray powder diffraction was employed. XRD pattern the sample quenched from 1350 °C showed only reflections typical of the orthophosphates YPO4 and Rb3Y(PO4)2. These results gave evidence for that Rb3Y2(PO4)3 was decomposed into these phosphates, which reveals its high-temperature instability. Accordingly, we found it necessary to examine the phase composition of Rb3Y2(PO4)3 above the temperature 1200 °C. Eight samples of the Rb3Y2(PO4)3 were prepared and placed into the furnace pre-heated to 1200 °C, and next each successively was heated to a higher and higher temperature of the series 1250, 1280, 1300, 1310, 1320, 1330, and 1340 °C. Each of seven samples had been heated at a given temperature for 10 min and then quenched. The eighth sample was cooled from 1340 °C to room temperature. The decomposition products appeared on the XRD pattern at 1330 °C. XRD pattern of 1340 °C showed only YPO4 and Rb3Y(PO4)2. These new phases alone remained on the XRD pattern of the sample (i.e., eighth) cooled down to room temperature.
On the basis of the above results of thermal and X-ray investigation it was assumed that the Rb3Y2(PO4)3 decomposes in the temperature range approximately from 1300 to 1330 °C according to the reaction scheme:
the decomposition reaction is irreversible.
The X-ray study of Rb3Y2(PO4)3 was performed using Siemens D5000 diffractometer (Brag-Brentano geometry, Ni filter, reflected pattern, CuKα radiation). Pattern was taken with 2θ step of 0.02°, in the 5°–61° range and the counting time of 10 s at each point. Basing on the literature data [10], concerning the structure types of A I3 B III2 (XVO4)3 compounds it was determined that the studied Rb3Y2(PO4)3 compound is isostructural with Rb3Yb2(PO4)3 compound (s.g. I213 (199), a = 1.687 nm). In order to refine lattice parameters, the Rietveld Profile Refinement method was exploited using Rietica software [30]. As a result of refining, the lattice parameter a = 1.70226 nm (with derived Bragg R factor = 0.61) was calculated for the studied Rb3Y2(PO4)3. Peak positions and integrated intensities are given in Table 1.
Taking into account the conditions of Rb3Y(PO4)2 and Rb3Y2(PO4)3 synthesis, their high-temperature instability like that of the Rb3PO4, in order to obtain equilibrium samples the YPO4–Rb3PO4 system was studies by using three series of samples. These samples were:
-
(1)
non-equimolar mixtures of YPO4 and Rb3Y2(PO4)3—to determine the phase equilibria within the composition range from 0 to 48.86 wt% Rb3PO4,
-
(2)
non-equimolar mixtures of Rb3Y2(PO4)3 and Rb3Y(PO4)2 orthophosphates—to determine the phase equilibria within the composition range from 48.86 to 65.6 wt% Rb3PO4,
-
(3)
non-equimolar mixtures of NH4H2PO4, dry Rb2CO3 and Rb3Y(PO4)2—to determine the phase equilibria within the composition range from 65.6 to 100 wt% Rb3PO4. The mixtures, after mixing and grinding in acetone, were dried at 120 °C and heated at 300 °C for 4 h, at 500 °C for 4 h, at 900 °C for 10 h, and at 1000 °C for 20 h (with intermediate grindings to insure a total reaction). The mixtures of the above items (1) and (2), after the mechanical processing, were synthesized via the solid state reaction by sintering at 1150 °C for 1 h. The sinters obtained were crushed and finely ground, to be finally investigated using thermoanalytical methods and X-ray powder diffraction.
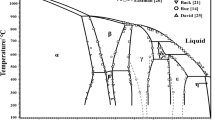
Based on these results, the phase diagram of the YPO4–Rb3PO4 system was constructed (Fig. 2). The points marked with circles concern temperatures at which thermal effects appeared on the DTA-heating curves. Notation for the composition range from 0 to about 55 wt% Rb3PO4 is as follows: (i) open circles (above 1250 °C) show temperatures of Rb3Y2(PO4)3 decomposition onset, (ii) filled circles show temperatures of full Rb3Y2(PO4)3 decomposition. The thermal effects accompanying the initial and final stages of Rb3Y2(PO4)3 decomposition gradually diminish to finally vanish. Liquidus curve could not be drawn in the above composition range because of the high-temperature instability of the intermediate compounds together with their complicated thermal dissociation. Also, we failed in an attempt to determine the melting points of the samples of composition range under discussion. Samples heated in the horizontal furnace (see “Experimental” section) vanished from the pyrometer field of view still before being fully melted, which appeared after cooling to room temperature. A lesser or larger partial melting of the samples was connected with their composition. Temperature that was read by using the optical pyrometer just in the moment of vanishing from the visual field is marked by x in Fig. 2.
In the Rb3PO4-rich part, of the composition range 48.86–100 wt% Rb3PO4, two eutectics occur. The eutectic mixture e1 melts at 1250 °C; its composition is 54 wt% Rb3Y(PO4)2 and 46 wt% Rb3Y2(PO4)3. The eutectic mixture e2 melts at 1225 °C; its composition is 36 wt% Rb3PO4 and 64 wt% Rb3Y(PO4)2. Polymorphic transformation α/β-Rb3PO4 in the pure compound occurs at the temperature of 1040 °C. In the composition range Rb3Y(PO4)2–Rb3PO4, the polymorphic transformation is recorded on DTA-heating curves as strong endothermal effects. Their onset is the temperatures range of 1010–1040 °C.
References
Hong HY-P, Chinn SR. Crystal structure and fluorescence lifetime of potassium neodymium orthophosphate, K3Nd(PO4)2, a new laser material. Mat Res Bull. 1976;11:421–8.
Salmon R, Parent C, Vlasse M, Le Flem G. The crystal structure of a new high -Nd- concentration laser material: Na3Nd(PO4)2. Mat Res Bull. 1978;13:439–44.
Melnikov PP, Kalinin VB, Efremov VA, Komisarova LN. Dvojnyje ortofosfaty redkozemelnych elementov (Gd–Lu), ittria i skandia z rubidium. Neorg Mater. 1981;17(8):1452–5.
Finke B, Wulff H, Schwarz L. Stöchiometrische Leuchtstoffe aus Mischkristallen von Phosphaten des Typs Me +3 Me3+(PO4)2. Z phys Chemie Leipzig. 1981;262(6):1152–4.
Sasum U, Kloss M, Rohmann A, Schwarz L, Haberland D. Optical properties of some rare earth and alkaline rare earth orthophosphates. J Lumin. 1997;72–74:255–6.
Schwarz L, Finke B, Kloss M, Rohmann A, Sasum U, Haberland D. Investigations on the electronic structure of double phosphates of the type M3RE(PO4)2 (RE = rare earths, lanthanides). J Lumin. 1997;72–74:257–9.
Areva S, Hölsä J, Kloss M, Lahtinen M, Lamminmäki R-J, Lastusaari M, Schwarz L, Valkonen J. Structural Modifications of Rb3RE(PO4)2 phases (RE = La, Gd, Y). Mater Sci Forum. 2001;378–381:644–8.
Ushakov SV, Navrotsky A, Farmer JM, Boatner LA. Thermochemistry of the alkali rare-earth double phosphates A3RE(PO4)2. J Mater Res. 2004;19(7):2165–75.
Collin G, Comes R, Boilot JP, Colomban P. Disorder of tetrahedra in Nasicon-type structure-I. Na3Sc2(PO4)3: structures and ion–ion correlations. J Phys Chem Solids. 1986;47(9):843–54.
Carvajal JJ, Parreu I, Solé R, Solans X, Diaz F, Aguilo M. Growth and structural characterization of Rb3Yb2(PO4)3: a new material for laser and nonlinear optical applications. Chem Mater. 2005;17:6746–54.
Wisniewski D, Wojtowicz AJ, Drozdowski W, Farmer JM, Boatner LA. Rb3Lu(PO4)2:Ce and Cs3Lu(PO4)2:Ce—new promising scintillator materials. Cryst Res Technol. 2003;38(3–5):275–82.
Aitasalo T, Guzik M, Szuszkiewicz W, Hölsä J, Keller B, Legendziewicz J. Properties of ytterbium and neodymium doped alkali metal yttrium double phosphates of the M3Y1-xLnx(PO4)2 type. J Alloys Compd. 2004;380:405–12.
Guzik M, Aitasalo T, Szuszkiewicz W, Hölsä J, Keller B, Legendziewicz J. Optical spectroscopy of yttrium double phosphates doped by cerium and praseodymium ions. J Alloys Compd. 2004;380:368–75.
Legendziewicz J, Guzik M, Szuszkiewicz W. Charge transfer and f–f emission of trivalent ytterbium observed in double phosphates MIMIII(PO4)2 (MI = Na, Rb; MIII = Lu, Y). J Alloys Compd. 2008;451:165–71.
Szulia S, Kołodziej HA, Szuszkiewicz W, Czupińska G. Dielectric relaxation in double potassium yttrium orthophosphate K3Y(PO4)2 and sodium yttrium orthophosphate Na3Y(PO4)2. J Non Cryst Solids. 2010;356:805–8.
Szuszkiewicz W, Znamierowska T. Phase equilibria in the system YPO4–Na3PO4. Themochim Acta. 1991;188:293–7.
Jungowska W, Znamierowska T. The system LaPO4–K3PO4. J Solid State Chem. 1991;95:265–9.
Szczygieł I, Znamierowska T. Phase equilibria in the system CePO4–Na3PO4. J Solid State Chem. 1991;95:260–4.
Czupińska G, Znamierowska T. The System YPO4–K3PO4. J Therm Anal. 1993;39:539–44.
Znamierowska T, Bandrowski Sz. Investigation of phase equilibria in the system Nd2O3–Na2O–P2O5. Quasibinary section NdPO4–Na3PO4. Pol J Chem. 2006;80:1731–5.
Szczygieł I, Matraszek A, Znamierowska T. Phase equilibria in the Ce2O3–K2O–P2O5 system. J Therm Anal Calorim. 2008;93(3):671–6.
Szczygieł I, Znamierowska T, Mizer D. Phase equilibria in the oxide system Nd2O3–K2O–P2O5. Solid State Sci. 2010;12:1205–10.
Aldred AT. Cell volumes of APO4, AVO4, and ANbO4 compounds, where A = Sc, Y, La-Lu. Acta Cryst. 1984;B40:569–74.
Szuszkiewicz W, Znamierowska T. Phase equilibria in the system Y2O3–P2O5. Pol J Chem. 1989;63:381–91.
Radomińska E, Znamierowska T, Szuszkiewicz W. Phase equilibria in the system Rb3PO4–Ba3(PO4)2. J Therm Anal Calorim. 2011;103:761–6.
Voronin VI, Berger IF, Proskurnina NV, Sheptyakov DV, Goshchitskii BN, Burmakin EI, Stroev SS, GSh Shekhtman. Crystal structure of the low-temperature forms of cesium and rubidium orthophosphates. Inorg Mater. 2008;44(6):646–52.
Hoppe R, Seyfert HM. Zur Kenntnis wasserfreier Orthophosphate der höheren Alkalimetalle: K3PO4, Rb3PO4, Cs3PO4. Z Naturforsch. 1973;28b:507–8.
Rouanet A, Serra JJ, Allaf K, Coutures J, Dexpert H. Décomposition thermique des orthophosphates de Lanthanides dans I′air à 1200–2200 °C. Schémas réactionnesls et caractérisation de nouvelles phases solides. Rev int hautes Tempér Réfract Fr. 1979;16:437–43.
Serra JJ, Coutures J, Rouanet A, Dexpert H, Garon G. Etude des familles d′oxyphosphates de lanthanides (Ln/P > 1) : synthèse, caractérisation et stabilité thermique. Rev int hautes Tempér Réfract Fr. 1978;15:287–313.
Hunter BA. Rietica—a visual Rietveld program. Comm Powder Diffr Newslett. 1998;20:21.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Szuszkiewicz, W., Radomińska, E., Znamierowska, T. et al. Phase equilibria in the YPO4–Rb3PO4 system. J Therm Anal Calorim 111, 63–69 (2013). https://doi.org/10.1007/s10973-011-2172-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2172-7