Abstract
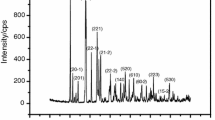
The thermal decomposition of synthetic serrabrancaite (MnPO4 · H2O) was studied in N2 atmosphere using TG-DTG-DTA. Thermal analysis results indicate that the decomposition occurs in two stages, which are assigned to the dehydration and the reduction processes and the final product is Mn2P2O7. X-ray powder diffraction, FT-IR and FT-Raman techniques were used for identification of the solid decomposition product. The decomposition kinetics analysis of MnPO4 · H2O was performed under non-isothermal condition through isoconversional methods of Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS). The dependences of activation energies on the extent of conversions are observed in the dehydration and the reduction reactions, which could be concluded the “multi-step” processes.



Similar content being viewed by others
References
Jouini A, Gâcon JC, Ferid M, Trabelsi-Ayadi M. Luminescence and scintillation properties of praseodymium poly and diphosphates. Opt Mater. 2003;24:175–80.
Kitsugi T, Yamamuro T, Nakamura T, Oka M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials. 1995;16:1101–7.
Jian-Jiang B, Dong-Wan K, Kug Sun H. Microwave dielectric properties of Ca2P2O7. J Eur Ceram Soc. 2003;23:2589–92.
Martinelli JR, Sene FF, Gomes L. Synthesis and properties of niobium barium phosphate glasses. J Non-Cryst Solids. 2000;263:299.
Carmen P, Josefina P, Regino SP, Caridad RV, Natalia S. Crystal growth, structure, and magnetic properties of a new polymorph of Fe2P2O7. Chem Mater. 2003;15:3347–51.
Marcu I-C, Sandulescu I, Yves Schuurman Y, Millet J-MM. Mechanism of n-butane oxidative dehydrogenation over tetravalent pyrophosphates catalysts. Appl Catal A. 2008;334;207–16.
Boyle FW Jr, Lindsay WL. Diffraction patterns and solubility products of several divalent manganese phosphate. Soil Sci Soc Am J. 1985;49:761–6.
Boyle FW Jr, Lindsay WL. Manganese phosphate equilibrium relationships in soils. Soil Sci Soc Am J. 1986;50:588–93.
Bagieu-Beucher M. Structure cristalline du polyphosphate de manganèse trivalent Mn(PO3)3. Acta Crystallogr. 1978;B34:1443–6.
Lightfoot P, Cheetham AK, Sleight AW. Structure of manganese(3+) phosphate monohydrate by synchrotron X-ray powder diffraction. Inorg Chem. 1987;26:3544–7.
Nietubyć R, Sobzak E, Attenkofeer KE. X-ray absorption fine structure study of manganese compounds. J Alloys Compd. 2001;328:126–31.
Durif A, Averbuch-Pouchot MT. Structure du diphosphate acide de manganèse(III): MnHP2O7. Acta Crystallogr. 1982;B38:2883–5.
Massa W, Yakubovich OV, Dimitrova OV. A novel modification of manganese orthophosphate Mn3(PO4)2. Solid State Sci. 2005;7:950–6.
Olbertz A, Stachel D, Svoboda I, Fuess H. Redetermination of the crystal structures of nickel cyclotetraphosphate, Ni2P4O12 and of cobalt cyclotetraphosphate, Co2P4O12. Z Kristallogr. 1995;210:241–2.
Stefanidis T, Nord AG. Structure studies of thortveitite-like dimanganese diphosphate, Mn2P2O7. Acta Crystallogr. 1984;C40:1995–9.
El-Bali B, Boukhari A, Glaum R, Gerk M, Maaß K. Contributions on Crystal Structures and Thermal Behaviour of Anhydrous Phosphates. XXIX Preparation and Structure Determination of SrMn2(PO4)2 and Redetermination of SrMn3(PO4)2. Z Anorg Allg Chem. 2000;626:2557–62.
Schneider S, Collin RL. Crystal structure of manganese pyrophosphate dihydrate Mn2P2O7·2H2O. Inorg Chem. 1973;12:2136–9.
Aranda MAG, Attfield JP, Bruque S. Study of manganese phosphate or arsenate hydrates, MnXO4·nH2O (X = P, As), phases and synthesis and structure of the simple, novel salt MnAsO4. Inorg Chem. 1993;32:1925–30.
Aranda MAG, Bruque S. Characterization of manganese(III) orthophosphate hydrate. Inorg Chem. 1990;29:1334–7.
Aranda MAG, Bruque S, Attfield JP. Crystal structures and characterization of a new manganese(III) arsenate, MnAsO4.1·2H2O and manganese(II) pyroarsenate, Mn2As2O7. Inorg Chem. 1991;30:2043–7.
Stojakovic D, Rajic N, Sajic S, Logar NZ, Kaucic V. A kinetic study of the thermal degradation of 3-methylaminopropylamine inside AlPO4-21. J Therm Anal Calorim. 2007;87:337–43.
Boonchom B, Youngme S, Srithanratana T, Danvirutai C. Synthesis of AlPO4 and kinetics of thermal decomposition of AlPO4·H2O-H4 precursor. J Therm Anal Calorim. 2007;91:511–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Kissinger HE. Reaction Kinetics in Differential Thermal Analysis. J Anal Chem. 1957;29:1702–6.
Boonchom B, Youngme S, Maensiri S, Danvirutai C. Nanocrystalline serrabrancaite (MnPO4·H2O) prepared by a simple precipitation route at low temperature. J Alloys Compd. 2006;454:78–82.
Witzke T, Wegner R, Doering T, PÖllmann H, Schuckmann W. Serrabrancaite, MnPO4·H2O, a new mineral from the Alto Serra Branca pegmatite, Pedra Lavrada, Paraiba, Brazil. Amer Mineral. 2000;85:847–9.
Baril M, Assaaoudi H, Butler IS. Pressure-tuning Raman microspectroscopic study of cobalt(II), manganese(II), zinc(II) and magnesium(II) yrophosphate dihydrates. J Mol Struc. 2005;751:168–71.
Harcharras M, Ennaciri A, Rulmont A, Gilbert B. Vibrational spectra and structures of double diphosphates M2CdP2O7 (M = Li, Na, K, Rb, Cs). Spectrochim Acta. 1997;A53:345–52.
Brouzi K, Ennaciri A, Harcharras M, Capitelli F. Structure and vibrational spectra of a new trihydrate diphosphate, MnNH4NaP2O7·3H2O. J Raman Spectrosc. 2004;35:41–6.
Vlaev LT, Nikolova MM, Gospodinov GG. Non-isothermal kinetics of dehydration of some selenite hexahydrates. J Solid State Chem. 2004;177:2663–9.
Vlase T, Vlase G, Brita N, Doca N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J Therm Anal Calorim. 2007;88:631–5.
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Calorim. 2007;88:805–12.
Budrugeac P. The Kissinger law and the IKP method for evaluating the non-isothermal kinetic parameters. J Therm Anal Calorim. 2007;89:143–51.
Singh BK, Sharma RK, Garg BS. Kinetics and molecular modeling of biologically active glutathione complexes with lead(II) ions. J Therm Anal Calorim. 2006;84:593–600.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Pyrolysis. 2008;81:253–62.
Zhang K, Hong J, Cao G, Zhan D, Tao Y, Cong C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Thermochim Acta. 2005;437:145–9.
Boonchom B, Maensiri S, Danvirutai C. Soft solution synthesis, non-isothermal decomposition kinetics and characterization of manganese dihydrogen phosphate dihydrate Mn(H2PO4)2·2H2O and its thermal transformation products. Mater Chem Phys. 2008;109:404–10.
Boonchom B. Kinetics and thermodynamic properties of the thermal decomposition of manganese dihydrogenphosphate dihydrate. J Chem Eng Data. 2008;53:1553–8.
Boonchom B, Danvirutai C. A simple synthesis and thermal decomposition kinetics of MnHPO4·3H2O rod-like microparticles obtained by spontaneous precipitation route. J Optoelectron Adv Mater. 2008;10:492–9.
Boonchom B, Danvirutai C. Thermal decomposition kinetics of FePO4·3H2O precursor to synthetize spherical nanoparticles FePO4. Ind Eng Chem Res. 2007;46:9071–6.
Boonchom B, Danvirutai C. Synthesis of MnNiP2O7 and nonisothermal decomposition kinetics of a new binary Mn0.5Ni0.5HPO4·H2O precursor obtained from a rapid coprecipitation at ambient temperature. Ind Eng Chem Res. 2008;47:5976–81.
Noisong P, Danvirutai C, Srithanratana T, Boonchom B. Synthesis, characterization and non-isothermal decomposition kinetics of manganese hypophosphite monohydrate. Solid State Sci. 2008;10:1598–604.
Acknowledgments
The authors would like to thank the Chemistry Department, Khon Kaen University for providing research facilities. This work is financially supported by the Thailand Research Fund (TRF) and the Commission on Higher Education (CHE): Research Grant for New Scholar (MRG5280073), Ministry of Science and Technology, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonchom, B., Danvirutai, C. & Thongkam, M. Non-isothermal decomposition kinetics of synthetic serrabrancaite (MnPO4 · H2O) precursor in N2 atmosphere. J Therm Anal Calorim 99, 357–362 (2010). https://doi.org/10.1007/s10973-009-0096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0096-2