Abstract
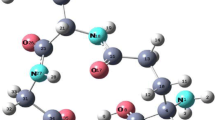
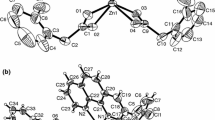
Lead(II) complexes of reduced glutathione (GSH) of general composition [Pb(L)(X)]·H2O (where L=GSH; X=Cl, NO3, CH3COO, NCS) have been synthesized and characterized by elemental analyses, infrared spectra and electronic spectra. Thermogravimetric (TG) and differential thermal analytical (DTA) studies have been carried out for these complexes. Infrared spectra indicate deprotonation and coordination of cysteinyl sulphur with metal ion. It indicates the presence of water molecule in the complexes that has been supported by TG/DTA. The thermal behaviour of complexes shows that water molecule is removed in first step-followed removal of anions and then decomposition of the ligand molecule in the subsequent steps. Thermal decomposition of all the complexes proceeds via first order kinetics. The thermodynamic activation parameters, such as E*, A, ΔH*, ΔS* and ΔG* have been calculated. The geometry of the metal complexes has been studied with the help of molecular modeling for energy minimization calculation.
Similar content being viewed by others
References
DN Kumar BS Garg (2002) J. Therm. Anal. Cal. 69 607 Occurrence Handle1:CAS:528:DC%2BD38Xms1eksrY%3D Occurrence Handle10.1023/A:1019976226610
R Sharma NK Kaushik (2004) J. Therm. Anal. Cal. 78 953 Occurrence Handle1:CAS:528:DC%2BD2cXhtVGmt7vJ
PC Jocelyn (1972) Biochemistry of the SH group Academic Press London
A Verma JM Simard JWE Worall VM Rotello (2004) J. Am. Chem. Soc. 126 13987 Occurrence Handle1:CAS:528:DC%2BD2cXot1Kqtb8%3D Occurrence Handle10.1021/ja046572r
KD Sugden DM Stearns (2000) J. Environ. Path Toxicol. Oncol. 19 215 Occurrence Handle1:CAS:528:DC%2BD3cXmvFKmtbw%3D
S Grabner J Kosmrlj N Bukovec M Cemazer (2003) J. Inorg. Biochem. 95 105 Occurrence Handle1:CAS:528:DC%2BD3sXjvV2nt74%3D Occurrence Handle10.1016/S0162-0134(03)00092-8
KP Rice PG Penketh S Krishnamurthy AC Sartorelli (2005) Biochem. Pharmcology 69 1463 Occurrence Handle1:CAS:528:DC%2BD2MXjs1Ckurw%3D Occurrence Handle10.1016/j.bcp.2005.02.016
WH Ang I Khalaila CS Allardyce L Juillerat-Jeanneret PJ Dyson (2005) J. Am. Chem. Soc. 127 1382 Occurrence Handle1:CAS:528:DC%2BD2MXjtFGmsA%3D%3D Occurrence Handle10.1021/ja0432618
S Kojima K Nakayama H Ishida (2004) J. Radiation Res. 45 33 Occurrence Handle1:CAS:528:DC%2BD2cXjvFygtrs%3D Occurrence Handle10.1269/jrr.45.33
B Ning C Wang F Morel S Nowell DL Ratnasinghe W Carter FF Kadlubar B Coles (2004) Pharmacogenetics 14 35 Occurrence Handle1:CAS:528:DC%2BD2cXjs1agsLw%3D Occurrence Handle10.1097/00008571-200401000-00004
Z Kopanski M Grabowska A Kosiniak-Kamysz J Bertrandt L Kolodziejski W Opoka M Schlegel-Zawadzka (2004) Biofactors 22 79 Occurrence Handle1:CAS:528:DC%2BD2cXhtFGns7zL
L Struzynska G Sulkowski A Lenkiewicz U Rafalowska (2002) Folia Neuro-pathologica 40 203 Occurrence Handle1:CAS:528:DC%2BD3sXktFKit7k%3D
K. Haraa, S. Ohmori, M. Nagano, Y. Kim and H. Miura, Proc. ICMR Semin. (1994) (Proceed of Asia-Pacific Symp. on Environ. and Occupational Health), (1993) 99.
G Li Y Jin M Zhao X Liu A Chem Z Xu (2003) Zhongguo Gongye Yixue Zazhi 16 163 Occurrence Handle1:CAS:528:DC%2BD2cXjvFGnurs%3D
AW Coats JP Redfern (1964) Nature 201 68 Occurrence Handle1:CAS:528:DyaF2cXjsFWltA%3D%3D
J Zsakó (1968) J. Phys. Chem. 72 2406 Occurrence Handle10.1021/j100853a022
PK Singh BS Garg DN Kumar BK Singh (2001) Ind. J. Chem. 40A 139
J Silver MY Hamed IEG Morrison (1985) Inorg. Chim. Acta 107 169 Occurrence Handle1:CAS:528:DyaL28Xhtl2qsA%3D%3D Occurrence Handle10.1016/S0020-1693(00)80699-4
G Domazetis RJ Magee BD James (1979) J. Organomet. Chem. 173 357 Occurrence Handle1:CAS:528:DyaE1MXlsFyjurY%3D Occurrence Handle10.1016/S0022-328X(00)84791-9
RM Silverstein OG Bassler TC Morrill (1974) Spectrophometric identification of organic compounds Wiley New York 108
H Shindo TL Brown (1965) J. Am. Chem. Soc. 87 1904 Occurrence Handle1:CAS:528:DyaF2MXoslCqtQ%3D%3D Occurrence Handle10.1021/ja01087a013
GB Deacon RJ Phillips (1980) Coord. Chem. Rev. 33 227 Occurrence Handle1:CAS:528:DyaL3MXjvVOqsw%3D%3D Occurrence Handle10.1016/S0010-8545(00)80455-5
GB Deacon F Huber RJ Phillips (1985) Inorg. Chem. Acta 104 41 Occurrence Handle1:CAS:528:DyaL2MXlvVKjtbw%3D Occurrence Handle10.1016/S0020-1693(00)83783-4
K Nakamoto (1978) Infrared and Raman spectra of inorganic and coordination compounds Wiley New York
VB Rana DP Singh P Singh MP Teotia (1981) Transition Met. Chem. 6 36 Occurrence Handle1:CAS:528:DyaL3MXitVWqtLc%3D Occurrence Handle10.1007/BF01143465
RA Bailey SL Kozak TW Michelsen WN Mills (1971) Coord. Chem. Rev. 6 407 Occurrence Handle1:CAS:528:DyaE3MXlsVGlt7s%3D Occurrence Handle10.1016/S0010-8545(00)80015-6
CNR Rao (1967) Ultraviolet and visible spectroscopy Butterworth London 190
K Nakamoto SJ McCarthy (1968) Spectroscopy and structure of metal chelate compounds John Wiley and Sons USA
S Akerfeldt G Lovgren (1964) Anal. Biochem. 8 223 Occurrence Handle1:CAS:528:DyaF2cXktVOrt7Y%3D Occurrence Handle10.1016/0003-2697(64)90050-8
H Bendiab J Meullemeetre MJ Schwing F Vierling (1982) J. Chem. Res. (S) 280
BR Curell (1969) Thermal Analysis Academic Press New York 1185
InstitutionalAuthorNameHyperchem (2005) Release 7.51 Professional version for window, Molecular Modeling system Hyperchem. Inc. Canada
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, B.K., Sharma, R.K. & Garg, B.S. Kinetics and molecular modeling of biologically active glutathione complexes with lead(II) ions. J Therm Anal Calorim 84, 593–600 (2006). https://doi.org/10.1007/s10973-005-7156-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7156-z