Abstract
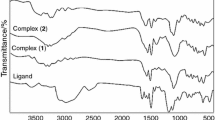
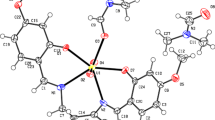
A complex of thiourea and bismuth trichloride has been synthesized. Its composition is Bi3Cl9[SC(NH2)2]7. Crystallographic data are a = 7.141(2) Å, b = 8.820(3) Å, c = 16.365(5) Å, α = 99.389(4)°, β = 95.422(4)°, γ = 106.177(4)°, triclinic system. There are the mononuclear anion [BiCl5SC(NH2)2]2− and the dinuclear cation {Bi2Cl4[SC(NH2)2]6}2+ with the Bi–Cl–Bi bridge bonds in the complex. The electric conductance of the absolute methanol solution contained the complex indicates that the complex is an ionic compound. Raman spectra indicate that the bismuth ion is coordinated by the sulfur atoms of the thiourea. The thermal analysis verifies the structure of complex. The TG–MASS curves show the structure rearrangement in the complex at about 118 °C. The DSC curves and calculation means that the structure rearrangement is irreversible.






Similar content being viewed by others
References
Lalia-Kantouri M, Manoussakis GE. Thermal Decomposition of Tris(N,N-Disubstituted Dithiocarbamate) Complexes of As(III), Sb(III) and Bi(III). J Therm Anal Cal. 1983;29:1151–69.
Lalia-Kantouri M, Christofides AG, Manoussakis GE. Thermal decomposition of tris(Piperidyldithiocarbamates) of As(Ill), Sb(lll) and Bi(lll). J Therm Anal Cal. 1984;29:279–95.
Sharutin VV, Egorova IV, Sharutina OK, Ivanenko TK, Adonin NY, Starichenko VF, et al. Tetranuclear bismuth complex Bi-4(O)(2)(O2CC6H2F3-3,4,5,)(8) · 2 eta(6)-C6H5Me: synthesis and structure. Russ J Coord Chem. 2003;29:838–44.
Cammi R, Lanfranchi M, Marchio L, Mora C, Paiola C, Pellinghelli M. Synthesis and molecular structure of the dihydrobis(thioxotriazolinyl)borato complexes of zinc(II), bismuth(III), and nickel(II). M ··· H-B interaction studied by ab initio calculations. Inorg Chem. A 2003;42:1769–78.
Goforth AM, Smith MD, Peterson L, Loye HCZ. Preparation and characterization of novel inorganic-organic hybrid materials containing rare, mixed-halide anions of bismuth(III). Inorg Chem. 2004;43:7042–9.
Balazs L, Breunig HJ, Lork E, Soran A, Silvestru AC. Isomers of a dibismuthane, R2Bi-BiR2 [R=2,6-(Me2NCH2)(2)C6H3], and unusual reactions with oxygen: formation of [R2Bi](2)(O-2) and R'R''Bi[R'=2-(Me2NCH2)-6-{Me2N(O)CH2}C6H3;R''=2-(Me2NCH2)-6-{O(O)C}C6H3]. Inorg Chem. 2006;45:2341–6.
Turner LE, Davidson MG, Jones MD, Ott H, Schulz VS, Wilson PJ. Bis(bismuth) toluene inverted-sandwich complex supported by aminetris(phenoxide) ligands. Inorg Chem. 2006;45:6123–5.
Goforth AM, Peterson L Jr, Smith MD, Loye HC. Syntheses and crystal structures of several novel alkylammonium iodobismuthate materials containing the 1,3-bis-(4-piperidinium)propane cation. J Solid State Chem. 2005;178:3529–40.
Jameson GB, Blaszó E, Oswald HR. Nitratopentakis(thiourea)bismuth(III) nitrate monohydrate, [Bi(NO3){SC(NH2)2}5](NO3)2·H2O, and trinitratotris (thiourea) bismuth(III), [Bi(NO3)3{SC(NH2)2}3]. Acta Crystallogr Sect C. 1984;40:350–4.
Bensch W, Blazsó E, Dubler E, Oswald HR. The structures of Bi2(CH3COO)6.3SC(NH2)2·H2O and Bi(CH3COO)3·3SC(NH2)2. Acta Crystallogr Sect C. 1987;43:1699–704.
Battaglia LP, Corradi AB, Pelizzi G, Tani MEV. Cationic and anionic bismuth(III) in chloro-thiourea complexes: crystal structure of [Bi(tu)6][Bi{(tu)1.5Cl1.5}Cl3]2 (tu = thiourea). J Chem Soc Dalton Trans. 1977;1141.
Bhat SG, Dharmaprakash SM. New metal-organic crystal: bismuth thiourea chloride. Mater Res Bull. 1998;33:833–40.
Williams DJ, Hutchings AM, McConnell NE, Faucher RA, Huck BE, Brevett CAS, et al. Main group metal halide complexes part XVIII: the synthesis, characterization, with sterically hindered thioureas and X-ray crystallographic study of a BiCl3 complex with 1-methyl-2(3H)-imidazolethione. Inorg Chim Acta. 2006;359:2252–5.
Vaira MD, Mani F, Stoppioni P. Lead(II) and bismuth(III) complexes with macrocyclic ligands. Eur J Inorg Chem. 1999;5:833–7.
Golovnev NN, Novikova GV, Vershinin VV, Churilov TD, Golovneva II. Complex formation of bismuth(III) with L-cysteine. Russ J Inorg Chem. 2003;48:1696–9.
Thompson KH, Orvig C. Boon and bane of metal ions in medicine. Science. 2003;300:936–9.
Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903.
Kopf-Maier P. Antitumor activity of some organometallic bismuth(III) thiolates. Inorg Chim Acta. 1988;152:49–52.
Cantos G, Barbieri CL, Iacomini M, Gorin PAJ, Travassos LR. Synthesis of antimony complexes of yeast mannan and mannan derivatives and their effect on Leishman-infected macrophages. Biochem J. 1993;289:155–60.
Kaloustian J, Pauli AM, Pieroni G, Portugal H. The use of thermal analysis in determination of some urinary calculi of calcium oxalate. J Therm Anal Cal. 2002;70:959–73.
Briand GG, Burford N. Bismuth compounds and preparations with biological or medicinal relevance. Chem Rev. 1999;99:2601–3657.
Ge RG, Sun HZ. Bioinorganic chemistry of bismuth and antimony: target sites of metallodrugs. Acc Chem Res. 2007;40:267–74.
Guo ZJ, Sadler PJ. Metals in medicine. Angew Chem Int Ed. 1999;38:1513–31.
Yu X, Zhang H, Cao Y, Chen Y, Wang Z. Synthesis and characteristics of a novel 3-D organic amine oxalate: (enH(2))(1.5)[Bi-3(C2O4)(6)(CO2CONHCH2CH2NH3) · 6.5H(2)O. J Solid State Chem. 2006;179:247–52.
Feldmann C. Preparation and crystal structure of [Bi3I(C4H8O3H2)(2)(C4H8O3H)(5)](2)-Bi8I30 containing the novel polynuclear [Bi8I30](6-) anion. J Solid State Chem. 2003;172:53–8.
Greenwood NN, Earnshaw A. Chemistry of the elements, chap. 7, 2nd ed. Oxford: Reed Educational and Professional Publishing Ltd; 1997. p. 553.
Jia RR, Yang YX, Chen YR, Jia YQ. Synthesis, crystal structure and thermal decomposition of solid complex. J Therm Anal Cal. 2004;76:157–63.
Zhong GQ, Luan SR, Wang P, Guo YC, Chen YR, Jia YQ. Synthesis, characterization and thermal decomposition of thiourea complexes of antimony and bismuth triiodide. J Therm Anal Cal. 2006;86:775–81.
Luan SR, Zhu YH, Jia YQ. Synthesis, characterization and thermal decomposition of alanine and taurine-salicylal schiff base complexes of magnesium. J Therm Anal Cal. 2009;95:951–6.
Madarász J, Pokol G. Comparative evolved gas analyses on thermal degradation of thiourea by coupled TG-FTIR and TG/DTA-MS instruments. J Therm Anal Cal. 2007;88:329–36.
Yamaguchi A, Penland RP, Mizushima S, Lane TJ, Curran C, Quagliano JV. Infrared absorption spectra of inorganic coordination complexes. XIV. Infrared studies of some metal thiourea complexes1. J Am Chem Soc. 1957;80:527.
Rivest R. Coordination complexes of titanium (IV) halides 111. Preparation and infrared spectra of the complexes of titanium, tetrachloride with urea, thiourea, and some of their derivatives. Can J Chem. 1962;40:2234–42.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, chap. 5–8, 5th ed. London: Wiley; 1978.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (20676038), the Key Project of Science and Technology for Ministry of Education (107045) and the Shanghai Leading Academic Discipline Project (Project Number: B502) for financial supports.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luan, S.R., Zhu, Y.H., Jia, Y.Q. et al. Characterization and thermal analysis of thiourea and bismuth trichloride complex. J Therm Anal Calorim 99, 523–530 (2010). https://doi.org/10.1007/s10973-009-0006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0006-7