Abstract
The complex compound bis(2,4-dichlorobenzoato-O)bis(thiourea-S)zinc(II) has been prepared and characterized by elemental analysis, thermal analysis, IR spectroscopy, and single-crystal X-ray analysis. During the thermal decomposition in inert atmosphere, thiourea, dichlorobenzene and carbon dioxide are evolved. The solid intermediate was confirmed by IR spectroscopy, and the final product of the thermal decomposition was proven by powder diffractometry. The coordination environment of the zinc(II) atom is built up by two sulphur atoms from two thiourea ligands and two oxygen atoms from two monodentate 2,4-dichlorobenzoate anions to form a distorted tetrahedral coordination around the zinc(II) atom (chromophore ZnO2S2). The mode of the carboxylate binding was assigned from the IR spectrum using the magnitude of the separation between the carboxylate stretches (Δ), and it is in good agreement with the crystal structure. The structure is also stabilized with hydrogen bonds of N–H···O and N–H···Cl type.
Similar content being viewed by others
Introduction
Zinc complexes are essential for many biological processes, including enzymatic catalysis. Zinc complexes prepared from organic acids have also been shown to have a variety of bioactive and biocatalytic functions [1]. Zinc carboxylates with N-donor and S-donor organic ligands have been extensively studied, and the relationship between their structure and reactivity has been well established [2]. Another constituent of the title compound, thiourea, is a well-known addition agent for copper plating, but it has also been shown to have analgesic, anti-inflammatory, antibacterial [3, 4], anticonvulsant, and cytotoxic activities [5]. From a structural perspective, thiourea is a monodentate ligand that coordinates to metals through its sulphur atom. Compounds containing thiourea are being studied in a variety of fields, including nonlinear optical crystals [6, 7], hybrid perovskites for photovoltaic devices [8], and precursors for gold nanoparticles [9]. Therefore, the thermal decomposition of the thiourea containing compounds can be important and can be found, e.g. in paper of Sankowska et al., Diaz et al. or our previous works, e.g. Krajníkova et al. [10,11,12]. It is also noteworthy to mention its use as a corrosion inhibitor [13]. Our previous works were focused mainly on the study of the spectral and biological properties as well as thermal stability and decomposition of the zinc carboxylates. As a part of our extensive study of zinc carboxylates over the last decades, an investigation of the relationship between composition of the carboxylates (e.g. functional groups and their position on the aromatic ring, presence of bioactive ligands) and their thermal, antimicrobial, and structural properties is taking place [14, 15]. Last ten years we mainly focused on benzoates, their halogeno- and hydroxyderivatives [16,17,18].
During the thermal decomposition of 2-aminobenzoatozinc(II) complexes, the release of 2-aminobenzaldehyde, aniline, and CO2 takes place. The final solid product of thermal analysis is zinc(II) oxide [19] By thermal decomposition of 3-aminobenzoatozinc(II) complex with thiourea ligand, [Zn(3-NH2C6H4COO)2tu2], the release of 2 mol of thiourea, 2 mol of aniline, with CO2 and CO takes place [20]. 4-aminobenzoato complex [Zn(4-NH2C6H4COO)2tu2]·H2O decomposed, after the release of crystal water molecule by mass loss of 3.52 %, to 2 mol of thiourea ligand with one mole of aniline. In the next step of thermal decomposition aniline, CO2 and CO liberated. ZnO is a product of thermal decomposition [21]. In the case of 4-hydroxyderivatives, we found in the product of the thermal decomposition organic ligand, phenol, CO2, and Zn as the final product of decomposition [18].
There are several structurally characterized aromatic zinc(II) carboxylates in which thiourea is involved. Compound [Zn(C6H4COO)2tu2]2 has molecular structure and contains two crystallographically independent molecules, where each zinc atom is tetrahedrally coordinated with two monodentate oxygen atoms from two benzoate anions and with two sulphur atoms from two thiourea ligands (chromophore ZnO2S2) [22]. In [Zn(2-BrC6H4COO)2tu2]·2H2O, the coordination environment of the central zinc atom with chromophore ZnO2S2 is a distorted tetrahedron created from two oxygen atoms of two monodentate coordinated 2-bromobenzoato ligands and further from two sulphur atoms from two thiourea ligands (chromophore ZnO2S2) [23]. It is also known crystal structure of several aliphatic zinc(II) carboxylates with thiourea from the literature [Zn(CH3COO)2tu2] [24]; [Zn(CH3CH2COO)2tu2] [25]; and [Zn(CCl3COO)2tu2]·H2O [26] with chromophore ZnO2S2.
In this paper synthesis, thermal, spectral properties, and crystal structure of new compound [Zn(2,4-Cl2C6H3COO)2tu2] are discussed.
Experimental
Synthesis and crystallization
The following chemicals of analytical grade were used: ZnCl2 (Fluka, Germany), Na2CO3 (Centralchem, Slovakia), 2,4-dichlorobenzoic acid (Aldrich, Germany), thiourea (Lachema, Czech Republic). An aqueous solution of ZnCl2 (1.36 g, 10 mmol) was added to an aqueous solution of Na2CO3 (1.06 g, 10 mmol) upon stirring. The freshly prepared precipitate of ZnCO3 was purified from sodium chloride by decantation, and its water suspension was added to an ethanol solution of 2,4-dichlorobenzoic acid (3.82 g, 20 mmol). Afterwards, thiourea (1.52 g, 20 mmol) was dissolved in 20 cm3 of ethanol and added dropwise to the mixture. The reaction mixture was stirred for two hrs at 313 K, and the solution was reduced in volume at 343 K in a water bath. The filtrate was left to stand at room temperature. Within a few days, colourless crystals were collected by filtration, washed with a small amount of cold ethanol, and then dried at room temperature (yield 84 %). The crystals suitable for X-ray experiment were separated manually under a microscope. All other experiments were carried out without separation of single crystals.
Instrumentation
Elemental analysis (C, H, N, S, Cl) was performed on a PerkinElmer 2400 CHN analyser (PerkinElmer, USA). The content of zinc was determined complexometrically using Complexone III as an agent and Eriochrome black T as an indicator. Analyses were calculated for C16H12Cl4N4O4S2Zn (Mr = 595.59) (found/calc.): C, 25.98/26.03 %; H, 3.19/3.28 %; N, 9.97/10.12 %; S, 11.24/11.58 %; Cl, 25.31/25.61 %; and Zn, 11.63/11.81 %.
IR spectrum was recorded on a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, USA) using KBr pellets (2 mg of sample per 200 mg of KBr) in the range 400–4000 cm–1.
The thermal measurements of TG/DTG and DTA were carried out up to 1173 K, heating rate at 9 K min–1 in nitrogen atmosphere by the NETZSCH STA 409 PC/PG thermoanalyser (Netzsch, Germany). The sample (amount 14.2 mg) was placed in ceramic crucible during the measurement.
The final solid product of thermal decomposition was identified with diffractometer Rigaku MiniFlex 600 (Rigaku, Japan), using β-filtered CuKα radiation (λ = 1.540593 Å), 40 kV/15 mA, in the range of 5°–60° 2Θ, step 0.02°.
X-ray crystallography
Data were collected at 173 K on Oxford Diffraction (Rigaku, Japan) Xcalibur diffractometer equipped with the Sapphire2 CCD area detector with a MoKα graphite-monochromated radiation (λ = 0.71073 Å) controlled by the CrysAlisPro software package [27], in the Θ range of 3.13°–26.49°. The final parameters R[F2 > 2σ(F2)], wR(F2) and S obtained from the last refinement were 0.021, 0.053, and 1.11, respectively. The structure was solved by direct methods using the program SHELXT [28] and refined by the full-matrix least-squares method on all F2 data using the program SHELXL 2018/3 [29]. Figures of the molecular and crystal structure were created using the software package Crystal Impact Diamond [30].
Results and discussion
The title compound [Zn(2,4-Cl2C6H3COO)2tu2] was characterized by elemental analysis, thermal analysis, IR spectroscopy, and X-ray analysis. The newly prepared complex is colourless, stable at light and air, and very soluble in hot water, methanol, ethanol, dimethylformamide, and dimethylsulfoxide. It is slightly soluble in chloroform, diethyl ether, and acetone, and insoluble in tetrachloromethane, toluene, and benzene.
Thermal analysis
The results of thermogravimetric analysis are presented in Fig. 1. In the first step, two moles of thiourea are evolved and then two moles of 1,3-dichlorobenzene are liberated (mass loss: exp. 73.80 %, calc. 74.93 %), which is accompanied by endothermic effect on the DTA curve at 493 K.
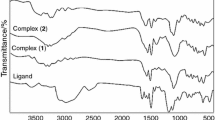
In the solid intermediate heated up to 493 K (see Fig. 2), the absorption bands of thiourea were missing (ν(N–H) = 3372, 3185 cm−1, ν(N–H) = 1608 cm−1, ν(C=S) = 1105 cm−1). The next step of the thermal decomposition in the temperature range 593–1023 K is the release of two moles of carbon dioxide (mass loss: exp. 16.30 %, calc. 14.78 %). This is accompanied by an exothermic effect at 993 K.
The final solid product of thermal decomposition was metallic zinc (exp. 9.90 %, calc. 10.98 %). The presence of metallic zinc was proven by recording powder diffraction data (Zn 2θ: 36.32°, 38.98° and 43.22°) (see Fig. 3). The obtained result corresponds to the powder pattern of metallic zinc found in the literature [31]. The following reaction is proposed for the decomposition process:
Complex compound with isonicotinamide ligand [Zn(2,4-Cl2C6H3COO)2inad2] decomposed similarly [32].
On other hand, the benzoatozinc(II) compounds with one Cl− substituent on aromatic ring in 2-position decomposed by evolving of bis(2-chlorophenyl)ketone (C6H4Cl)2CO, CO2, and final solid product of thermal decomposition are ZnO [17]. Thermal decomposition of 2-bromobenzoatozinc(II) complexes [16] and 4-bromobenzoato complex with thiourea ligand is similar. They decomposed also by evolving of thiourea ligand, bromophenyl ketone (C6H4Br)2CO, and CO2 [12]. The final solid product of thermal decomposition is ZnO. By thermal decomposition of 4-hydroxybenzoatozinc(II) compound with thiourea ligand,
Zn(4-OHC6H4COO)2tu2], in the first step of decomposition two moles of thiourea with one mole of phenol liberated and in the second step one mole of phenol and two moles of CO2 take place. The final solid product of thermal decomposition is metallic zinc [18].
IR spectroscopy
The IR spectrum confirmed the presence of functional groups of organic ligand (Fig. 4). The stretching vibrations of the thiourea N–H groups were found at 3372 and 3185 cm–1. In the case of carboxylate compounds, the values of the asymmetric and symmetric vibrations of carboxylate groups are important. The absorption band observed at 1589 cm−1 was assigned as νas(COO−) and that at 1358 cm−1 as νs(COO−). Sodium salt of 2,4-dichlorobenzoic acid shows asymmetric stretching vibrations νas(COO−) at 1589 cm−1 and symmetric stretching vibrations νs(COO−) at 1398 cm−1. The carboxylate binding modes were assigned from IR spectra using the magnitude of the separation between the carboxylate stretches (Δexp = νas—νs). The following order has been proposed for divalent metal carboxylates [33]: Δ (chelating) < < Δ (bridging) < Δ (ionic) < < Δ (monodentate). The Δ value observed for [Zn(2,4-Cl2C6H3COO)2tu2] (231 cm−1) is higher than the value for sodium salt Na(2,4-Cl2C6H3COO) (191 cm−1) that agrees with the monodentate coordination mode of carboxylate groups in the compound. IR spectroscopy can also provide information regarding whether nitrogen or sulphur atom of thiourea is involved in coordination. In the case when the sulphur atom of thiourea is the donor atom, the stretching vibration of ν(C=S) is shifted to lower frequency compared to free thiourea. In titled compound ν(C=S) appears at 1105 cm−1. Compared with free thiourea at 1164 cm−1, the ν(C=S) shift to lower wavelengths is apparent, and thus, the ligand coordination occurring via sulphur atom was assumed. This conclusion was later confirmed by single-crystal X-ray structure determination.
Structural analysis
The [Zn(2,4-Cl2C6H3COO)2tu2] compound crystallized with monoclinic lattice in centrosymmetric space group P2/n, with the parameters of the unit cell a = 10.5372(3) Å, b = 8.3984(2) Å, c = 12.9576(4) Å, and = 101.903(3)°. The geometry around the zinc(II) atom is close to tetrahedral, created by two oxygen atoms from two monodentate coordinated dichlorobenzoate anions, and two sulphur atoms from two thiourea molecules (Fig. 5). The solid-state molecule possesses an exact twofold symmetry passing through the central zinc atom; the symmetrically dependent atoms of the complex are obtained after this crystallographic twofold axis is applied. The distortion of the tetrahedron around zinc atom is the consequence of unequal ionic radii of the coordinating sulphur and oxygen ions of both coordinated ligands.
The mean value for Zn–O and Zn–S bond length (1.9790(10) and 2.3347(4) Å, respectively) does not deviate significantly from those observed for similar monomeric carboxylatozinc(II) complexes with thiourea: [Zn(2-BrC6H4COO)2tu2]·2H2O (2.0112(18) and 2.3256(8) Å, respectively) [23]; [Zn(C6H5COO)2tu2] (1.964(2) and 2.3673(14) Å, respectively) [22]. Additional Zn···O distances to the non-coordinated carboxylate oxygen atoms are 2.8127(12) Å (2 ×), and the highest value of tetrahedral angles X–Zn–Y is 112.73(3)°. The corresponding values for similar complexes mentioned above with chromophore ZnO2S2 (in the range 2.829(2)–3.282(7) Å; 119.3(3)–126.6(2)°, respectively) are comparable with the obtained data. The structure is stabilized by system of intramolecular as well as intermolecular hydrogen bonds of N–H···O type and by intermolecular hydrogen bonds of N–H···Cl type (Fig. 6). Hydrogen bonds of N–H···O type involve amino group of thiourea ligands and oxygen atom from carboxylate groups of the anionic ligands of the same molecule making hydrogen bonds’ motif of S11(6) type (S in Fig. 6). Also, interaction between hydrogen atom from amino group and oxygen atom from neighbouring molecule’s anionic ligand makes the similar motif of R21(6) type (R in Fig. 6), which lead to creation of polymeric network (Fig. 6). The second type of hydrogen bonds N–H···Cl (mean value for H···Cl distance is 3.8295 Å) is from amino group of the thiourea and chlorine from 2,4-dichlorobenzoato ligands.
Conclusions
During the thermal decomposition of studied compound, the thiourea, 1,3-dichlorobenzene, and carbon dioxide are evolving. The final product of thermal decomposition was metallic zinc, and that was confirmed by X-ray powder diffraction method.
The products of thermal decomposition are different, and it depends on structure of the complexes, type of decarboxylation, position of the substituent on the aromatic ring and mesomeric and inductive effect of the substituents. In the case of halogenoderivatives, if there are two chlorine atoms in the 2- and 4-positions on the benzene ring, or in the case of 4-hydroxyderivatives, thermal decomposition takes place with the release of 1-,3-dichlorobenzene or phenol, respectively, 2 mol of CO2 and the final product is metallic zinc.
If there is only one chlorine atom in the ortho-position, or bromine atom in the ortho-, or para-position as a substituent on the benzene ring, a halogenoketone and CO2 are always formed. The final product is ZnO. In the case of 2-aminoderivatives aldehyde, aniline and CO2 are evolved. 3-,4-aminoderivates allowed to develop aniline, CO2, and CO. Solid product of thermal decomposition is ZnO.
The coordination environment around zinc(II) atom is tetrahedral with chromophore ZnO2S2. Oxygen atoms from dichlorobenzoate anions are monodentate coordinated that is in a good agreement with the results of IR spectroscopy.
References
Győryová K, Szunyogová E, Kovářová J, Hudecová D, Mudroňová D. Biological and physicochemical study of zinc(II) propionate complexes with N-donor heterocyclic ligands. J Therm Anal Calorim. 2003;72:587–96.
Reinoso DM, Damiani DE, Tonetto GM. Zinc carboxylic salts used as catalyst in the biodiesel synthesis by esterification and transesterification: study of the stability in the reaction medium. Appl Catal A-Gen. 2012;449:88–95.
Alagarsamy V, Muthukumar V, Pavalarani N, Vasanthanathan P, Revathi R. Synthesis, analgesic and anti-inflammatory activities of some novel 2,3-disubstituted quinazolin-4(3H)-ones. Biol Pharm Bull. 2003;26:557–9.
Alagarsamy V, Murugananthan G, Venkateshaperumal R. Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-methyl-3-substituted quinazolin-4-(3H)-ones. Biol Pharm Bull. 2003;26:1711–4.
Suresha GP, Suhas R, Kapfo W, Gowda DC. Urea/thiourea derivatives of quinazolinoneelysine conjugates: Synthesis and structureeactivity relationships of a new series of antimicrobials. Eur J Med Chem. 2011;46:2530–40.
Sangeetha MK, Mariappan M, Madhurambal G, Mojumdar SC. TG-DTA, XRD, SEM, EDX, UV, and FT-IR spectroscopic studies of l-valine thiourea mixed crystal. J Therm Anal Cal. 2015;119(2):907–13.
Subashini A, Rajarajan K, Sagadevan S, Singh P, Podder J. Preparation and characterization of a bis thiourea sodium iodide (BTSI): A potential NLO crystal. J Therm Anal Cal. 2018;131(3):2179–86.
Nan-Nan X, Shuai-Kang Y, Xuan Z, Zheng-Zhen T, Qin-Yu Z, Jie D. Perfect self-assembling of one-dimensional lead iodides with tetrahedral Cu4I6S4 clusters: a high-symmetry cubic packing. Inorg Chem. 2019;58(4):2248–51.
Kossmann A, Ehnert R, Preuß A, Rüffer N, Korb M, Schulze S, Tegenkamp C, Köster F, Lang H. The di(thiourea)gold(I) complex [Au{S=C(NH2)2}2][SO3Me] as a precursor for the convenient preparation of gold nanoparticles. Z Naturfor B. 2020;75:239–49.
Sankowska M, Gajek A, Celiński M, Sałasińska K. Determination of gaseous products of thermal degradation of thiram. J Therm Anal Cal. 2017;128(3):1639–47.
Díaz M, Palop JA, Sanmartín C, Lizarraga E. Thermal stability and decomposition of urea, thiourea and selenourea analogous diselenide derivatives. J Therm Anal Cal. 2017;127(2):1663–74.
Krajníková A, Győryová K, Kovářová J, Hudecová D, Hubáčková J, Nour El-Dien FA, Koman M. Thermoanalytical, spectral and biological study of 4-bromobenzoatozinc(II) complexes containing bioactive organic ligands. J Therm Anal Calorim. 2012;110:177–85.
Mathad GS. Copper Interconnets, In: New Contact Metallurgies/Structures, and Low-K Interlevel Dielectrics. The Electrochemical Society. Pennington: New Jersey; 2001.
Erdélyiová A, Győryová K, Gyepes R, Halás L, Kovářová J. Synthesis, spectral, thermal and structural study of bis(2-bromobenzoato-O, O′)-bis(methyl-3-pyridylcarbamate-N)-zinc(II). Polyhedron. 2009;28:131–7.
Vargová Z, Zeleňák V, Cı́sařová I, Xxx K. Correlation of thermal and spectral properties of zinc(II) complexes of pyridinecarboxylic acids with their crystal structures. Thermochim Acta. 2004;423:149–57.
Krajníková A, Győryová K, Hudecová D, Kovářová J, Vargová Z. Thermal decomposition and antimicrobial activity of zinc(II) 2-bromobenzoates with organic ligands. J Therm Anal Calorim. 2011;105:451–60.
Findoráková L, Győryová K, Hudecová D, Mudroňová D, Kovářová J, Homzová K, Nour El-Dien FA. Thermal decomposition study and biological characterization of zinc(II) 2-chlorobenzoate complexes with bioactive ligands. J Therm Anal Calorim. 2013;111:1771–81.
Homzová K, Győryová K, Bujdošová Z, Hudecová D, Ganajová M, Vargová Z, Kovářová J. Synthesis, thermal, spectral and biological properties of zinc(II) 4-hydroxybenzoate complexes. J Therm Anal Calorim. 2014;116:77–91.
Krajníková A, Rotaru A, Győryová K, Homzová K, Manoela HO, Kovářová J, Hudecová D. Thermal behaviour and antimicrobial assay of some new zinc(II) 2-aminobenzoate complex compounds with bioactive ligands. J Therm Anal Calorim. 2015;120:73–83.
Smolková R, Smolko L, Györyová K, Homzová K, Tomčík P, Hudecová D, Findoráková L. New zinc (II) 3-aminobenzoates with bioactive ligands: synthesis, thermal, spectral and antimicrobial properties. Thermochim Acta. 2018;669:1–7.
Homzová K, Györyová K, Hudecová D, Koman M, Melnik M, Kovářová J. Synthesis, thermal, spectral, and biological properties of zinc(II) 4-aminobenzoate Complexes. J Therm Anal Calorim. 2017;129:1065–82.
Černák J, Adzimová I, Gérard F, Hardy AM. Bis(benzoato-O)bis(thiourea-S)zinc(II). Acta Crystallogr C. 1995;51:392–5.
Krajníková A, Gyepes R, Győryová K, Šubrt J, Imrich J. Preparation, crystal structure and spectroscopic properties of dimeric [Zn(2bromobenzoato)2 (phenazone)]2 and monomeric Zn(2-bromobenzoato)2(thiourea)2]·2H2O. J Chem Crystallogr. 2009;41:1036–43.
Cavalca L, Gasparri GF, Andreetti D, Domiano P. The crystal structure of bisthiourea-zinc acetate. Acta Crystallogr. 1967;22:90–8.
Smolander K, Ahlgrén M, Melník M, Skoršepa J, Györyová K. Monomeric (dipropionato-O)(dithiourea-S)zinc(II). Acta Crystallogr C. 1994;50:1900–2.
Potočňák I, Dunaj-Jurčo M, Petříček V, Černák J. Bis(thiourea-[kappa]S)bis(trichloroacetato-[kappa]O)zinc(II) monohydrate. Acta Crystallogr C. 1994;50:1902–4.
Rigaku Oxford Diffraction. CrysAlis PRO, Version 1.171.39.46; 2018.
Sheldrick GM. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr A. 2015;71:3–8.
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr C. 2015;71:3–8.
Brandenburg K. Diamond. Bonn: Crystal Impact GbR; 2006.
Swanson T. Standard X-ray diffraction powder patterns. Natl Bur Stand (US) Circ. 1953;539(I):16.
Homzová K, Győryová K, Koman M, Melník M, Juhászová Ž. Synthesis, crystal structure and spectroscopic and thermal properties of the polymeric compound catena-poly [bis(2,4-dichlorobenzoato)zinc(II)-isonicotinamide]. Acta Crystallogr C. 2015;71:814–9.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 5th ed. New York: Wiley; 1997.
Acknowledgements
Authors are thankful to Professor Vladimír Zeleňák for thermal measurements.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This research was funded by the Slovak Grant Agencies APVV (Proj. No. APVV-18-0016) and VEGA (Proj. No. 1/0189/22 and 1/0128/21). The financial support is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuchár, J., Homzová, K., Tomčík, P. et al. Synthesis, spectral, thermal properties, and crystal structure of bis(2,4-dichlorobenzoato-O)bis(thiourea-S)zinc(II). J Therm Anal Calorim (2023). https://doi.org/10.1007/s10973-023-12653-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-023-12653-9