Abstract
Uranyl(VI) complexes with unsymmetrical N2O2 Schiff base ligands were synthesized and characterized. Their characterization was performed using UV-Vis, 1H NMR, cyclic voltammetry, single-crystal X-ray crystallography, IR, TG and C.H.N. techniques. X-ray crystallography of the complexes show that beside coordination of the tetradentate Schiff base, one DMF molecule is also coordinated. In order to investigate the effect of the substitutional groups of the Schiff base on the oxidation and reduction potentials, we used the cyclic voltammetry method. Electrochemistry of these complexes showed that the presence of electron releasing groups accelerates oxidation of the complexes. The kinetics of thermal decomposition was studied using thermal gravimetric method (TG) and Coats-Redfern equation. According to Coats-Redfern plots, the kinetics of thermal decomposition of the studied complexes is first-order in all stages. Also the kinetics and mechanism of the exchange reaction of the coordinated solvent with tributylphosphine was carried out in solution, using spectrophotometric method. As a result, the second order rate constants at four temperatures and the activation parameters were calculated showing an associative mechanism for all corresponding complexes. It was concluded that the steric and the electronic properties of the complexes influence the reaction rate significantly.

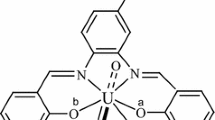
X-ray crystallography of the Uranyl (VI) complexes shows that besides coordination of the tetradentate Schiff base, one DMFmolecule is also coordinated.






Similar content being viewed by others
References
Vaughn A E, Bassil D B, Barnes C L, Tucker S A and Duval P B 2006 J. Am. Chem. Soc. 128 10656
Mihalcea I, Henry N, Clavier N, Dacheux N and Loiseau T 2011 Inorg. Chem. 50 6243
Riisiö A, Väisänen A and Sillanpää R 2013 Inorg. Chem. 52 8591
Ghosh S, Biswas S, Bauzá A, Barceló-Oliver M, Frontera A and Ghosh A 2013 Inorg. Chem. 52 7508
Nocton G, Horeglad P, Vetere V, Pécaut J, Dubois L, Maldivi P, Edelstein N M and Mazzanti M 2010 J. Am. Chem. Soc. 132 495
Brancatelli G, Pappalardo A, Sfrazzetto G T, Notti A and Geremia S 2013 Inorg. Chim. Acta 396 25
Shen X, Liao L, Chen L, He Y, Xu C, Xiao X, Lin Y and Nie C 2014 Spectrochim. Acta Part A 123 110
Mandal L, Bhattacharya S and Mohanta S 2013 Inorganica Chimica Acta 406 87
Back D F, Manzoni de Oliveira G, Roman D, Ballin M A, Kober R and Piquini P C 2014 Inorg. Chim. Acta 412 6
Takao K and Ikeda Y 2007 Inorg. Chem. 46 1550
Wu X, Bharara M S, Bray T H, Tate B K and Gorden A E V 2009 Inorganica Chim Acta 362 1847
Bharara M S, Strawbridge K J, Vilsek Z, Bray T H and Gorden A E V 2007 Inorg. Chem. 46 8309
Takao K, Tsushima S, Takao S, Scheinost A C, Bernhard G, Ikeda Y and Hennig C 2009 Inorg. Chem. 48 9602
Mizuoka K, Tsushima S, Hasegawa M, Hoshi T and Ikeda Y 2005 Inorg. Chem. 44 6211
Takao K, Kato M, Takao S, Nagasawa A, Bernhard G, Hennig C and Ikeda Y 2010 Inorg. Chem. 49 2349
Takao K, Takahashi T and Ikeda Y 2009 Inorg. Chem. 48 1744
Sessler J A, Melfi P J and Pantos G D 2006 Coor. Chem. Rev. 250 816
Kannappan R, Tooke D M, Spek A L and Reedijk J 2006 Inorg. Chim. Acta 359 334
Arnold P L, Blake A J, Wilson C and Love J B 2004 Inorg. Chem. 43 8206
Takao K, Kato M, Takao S, Nagasawa A, Scheinost A C, Bernhard G, Hennig C and Ikeda Y 2009 Actinides 1
Schettini M F, Wu G and Hayton T W 2009 Inorg. Chem. 48 11799
Mizuoka K and Ikeda Y 2003 Inorg. Chem. 42 3396
Evans J D, Junk P C and Smith M K 2002 Polyhedron 21 2421
Agilent Technologies (2012) Crys Alis PRO. Yarnton, Oxfordshire, England
Palatinus L and Chapuis G 2007 J. Appl. Cryst. 40 786
Petricek V, Dusek M and Palatinus L (2006) Jana 2006 Structure Determination Software Programs. Institute of Physics, Praha, Czech Republic
Costa G, Mestroni G, Tauzher G and Stefani L 1966 Organometallic Chem. 6 181
Garg B S and Kumar D N 2003 Spectrochim. Acta Part A 59 229
Anthonysamy A and Balasubramanian S 2005 Inorg. Chem. Commun. 8 908
Kumar D N and Garg B S 2006 Spectrochim. Acta Part A 59 141
Asadi M and Sarvestani A H 2001 Can. J. Chem. 79 1360
Asadi M and Sarvestani A H 2002 J. Chem. Research(s) 520
Coats AW and Redfern J P 1964 Nature 201 68
Aravindakshan K K and Muraleedharan K 1989 Thermochim. Acta 155 247
Nair M K M and Radhakrishnan P K 1995 Thermochim. Acta 261 141
Mathew S, Nair C G R and Ninan K N 1989 Thermochim. Acta 155 247
Acknowledgements
We are grateful to Shiraz University Research Council for its financial support. The project P204/11/0809 of the Grant agency of the Czech Republic supported the crystallographic part of the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC 914883 (3-OMe) and 949507 (4-OMe) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ASADI, Z., ASADI, M., ZEINALI, A. et al. Synthesis, structural investigation and kinetic studies of uranyl(VI) unsymmetrical Schiff base complexes. J Chem Sci 126, 1673–1683 (2014). https://doi.org/10.1007/s12039-014-0720-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0720-y