Abstract
Since the discovery of its photocatalytic properties, titanium dioxide has remained one of the most popular and widely used metal oxide photocatalysts. Its major drawback, however, lies in the narrow region (UV) of sunlight necessary to produce reactive oxygen species. This have been countered by sensitizing with organic dyes to red-shift the absorption spectrum but also with doping of other metals and non-metals. Volume doping or surface modification have demonstrated improved photocatalytic efficiency, mainly via red-shifted absorption by introduction of intermediate energy states between the valence band (VB) and conduction band (CB) and increased number of surface hydroxyl groups (which can form reactive hydroxyl radicals) from charge compensation, and in some cases by improved surface-adsorption of organic molecules. Doped titania and complex titanates have traditionally been produced via, for instance, co-precipitation of mixed metal salts or via solid-state synthesis. While these methods usually are simple, they offer limited control over size, shape, and phase composition. An alternative is the use of single-source precursors (SSPs), i.e., molecules already containing the desired metal ratio in a homogenous distribution. The last one or two decades have seen an increased number of reported transition metal-doped titanium oxo-alkoxides (TOA), particularly for the first-row transition metals as potential single-source precursors (SSP) for doped titania and complex titanates. This review aims at providing an overview of TM-doped TOAs, focusing on first and second row TM elements, with special emphasis on their synthesis, photochemical properties, and their applications as SSPs.

Graphical abstract
Highlights
-
The synthesis and structures of transition metal-doped titanium oxo-alkoxy complexes are reviewed.
-
Their applications as precursors for complex oxide materials are reported with focus on photo(electrochemical) applications.
-
An extensive set of references is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium(IV) dioxide (TiO2, titania) has been in the spotlight for photochemical reactions since Honda and Fujishima reported the ability of titania to photochemically split water in 1972 [1]. Many comprehensive reviews on the synthesis and applications of titania nanoparticles have been published over the years (e.g., refs. [2,3,4,5,6,7,8]). It is also employed as anode material in dye-sensitized solar cells [9]. The two major allotropes of titania, anatase and rutile, possess different photocatalytic activities, where anatase despite its wider band gap (ca. 3.2 eV) is considered more effective than rutile (ca. 3.0 eV). This have been explained, for instance, by lower charge carrier recombination rates and higher density of surface hydroxyl groups for the former phase [10, 11]. The photoexcitation of titania results in an electron-hole pair where the excited electron can react with oxygen to form the super oxide radical or with terminal hydroxide groups to surface hydroxyl radicals, and the hole can reduce water to hydroxyl radicals. These radical species have potential to completely mineralize organic pollutants [12].
Below ca. 14 nm, anatase is the most stable phase [13], but over this size rutile becomes the thermodynamically most stable phase. Pure anatase will irreversibly transform into rutile between 500 °C and 600 °C. The transformation is initiated from the particle surface [14]. Hence, surface functionalization, i.e. dopants, have the ability to stabilize the surface and increase the onset temperature of rutile transformation and also hinders aggregation into larger particles with reduction of active surface area. Additionally, the incorporation of surface lanthanides (oxides) on titania have facilitated photocatalytic degradation via improved surface adsorption of organic pollutants (Lewis acid-base complex formation) and formation of intermediate energy levels between VB and CB [15]. Conventional wet chemical methods to surface modify/dope titania with TMs include precipitation of metal salts on pre-formed nanoparticles [16], sol-gel synthesis [17,18,19,20], and hydro/solvothermal processing [21,22,23].
Several successful efforts to synthesize single-source precursors for bimetallic titanates have been reported including cobalt titanate [24], titanium molybdate [25], and zirconium titanate [26]. Furthermore, additional organic ligands in the SSPs can facilitate formation of highly porous materials due to gas evolution during combustion/calcination [27] and also increase volatiliy for application in chemical vapor deposition (CVD) of complex oxides [28]. Organosoluble metal doped TOAs have additionally been proposed as “molecular photocatalysts” in synthetic organic chemistry, thus their absorption in the visible region is of interest [29]. Metal-doped and complex titanates are likely to find advanced applications in (photo)catalysis and biomedicine, and their controlled synthesis via carefully designed single-source precursors remains attractive.
The last twenty years have seen an increased interest in TOAs doped with transition metals, particularly the first and second row elements. The purpose of this review is to provide an overview of the synthetic strategies employed to produce TM-doped TOAs, types of structures obtained, and their applications which special emphasis on photochemistry and the use as SSPs. The scope is limited to complexes containing Ti-O(R)-M linkages (M = first or second row transition metal, R = alkoxide, or bridging ligand) and where titanium(IV) alkoxides have been used as the titanium precursor to keep it relevant to the sol-gel process and formation of doped titania and titanates. Herein the term titanium oxo-alkoxy coordination complex (or simply complex) is used and “cluster” is reserved to compounds with metal-metal bonds as originally proposed by F.A. Cotton [30].
2 Formation of titania nanoparticles
For sol-gel reactions in organic medium, titanium(IV) alkoxides are the most common precursors. Titanium alkoxides with less bulky side-groups tend to be oligomeric, e.g., [Ti(OEt)4]4 and [Ti(OMe)4]4 [31, 32] while [Ti(iOPr)4] tends to be monomeric in non-polar solvents [33]. As a result of the lower electronegativity of titanium compared to silicon and coordination unsaturation, titanium alkoxides reacts more rapidly with water than the silicon alkoxides [34,35,36]. It is well-known that addition of water to pure titanium alkoxides immediately results in a white precipitate, which is usually not the case for titanium alkoxides modified with chelating ligands. This have been interpreted as stabilization against of hydrolysis by, e.g., blocking of coordination sites from H2O and by stabilization from ligands not easily hydrolysable [37, 38]. However, strongly complexing ligands have been demonstrated less hydrolysable compared to alkoxy groups and can thus provide both protection against aggregation and to control the size of primary titania nanoparticles [39, 40]. Studies of acetate-modified titanium(IV) butoxide by Doeuff et al. [41] demonstrated the bridging acetate to be more easily hydrolyzed compared to the chelating acetate, where the latter one needed thermal treatment for removal. The strongly chelating acetate increased gelation time, favoring gels rather than colloidal particles.
Extensive efforts have been devoted on the functionalization of titanium oxo-alkoxy compounds with different organic ligands which is an attractive way to introduce new functions and create organic-inorganic hybrid materials. This have been covered in several comprehensive reviews [35, 42,43,44]. Hydrolysis of ligand-modified TOAs has been used to obtain titania of complex morphologies [45, 46].
More recent studies by Kessler and co-workers [34], however, have actually demonstrated the increased reactivity of different metal alkoxides modified with chelating ligands, based on increased M–O bond lengths and calorimetric measurements. A series of studies have reported the hydrolysis of heteroleptic titanium oxo-alkoxide complexes modified with chelating and bridging ligands in contact with water to form crystalline nano-anatase [45, 47]. Due to the high reactivity of HO-Ti-OR, these species are never observed as a product of hydrolysis analogous to HO-Si-OR3, but instead oligonuclear titanium oxo-alkoxy complexes with hexacoordinated titanium centers are formed as discrete TiaOb(OR)cLd species in a simultaneous hydrolysis-condensation reaction [48]. This have recently been supported by in situ studies by Kanaev’s group [49, 50]. These oxo-species will act as nuclei for the formation of primary metal oxide particles via aggregation and condensation in a process referred to as micelles templated by self-assembly of ligands (MTSAL) (Fig. 1). The ligands from the oxo-complexes are transferred to the particle surface and provide solution stability, forming a colloid. The strength and kinetics of the nanoparticle-ligand interactions can be studied employing diffusion ordered nuclear magnetic resonance spectroscopy [51], as well as distinguish between distinct chemical species in solution [52, 53]. The formation of an “invisible” colloid stabilized by chelating and/or bridging ligands presumably supported the idea of inhibited hydrolysis by modification with chelating ligands.
Formation of MTSALs from the hydrolysis and condensation of molecular precursors into oligonuclear oxo-complexes that subsequently aggregate and condense into nuclei, stabilized in solution by surface ligands. Reproduced from ref. [109]
Nano-anatase can form directly from single crystals of a titanium oxo-alkoxide via thermolysis or (thermal) hydrolysis. For example, immersing crystals of [Ti(OCH3)4]4 in boiling water resulted in fully crystalline anatase nanoparticles after 30 min. Hydrolysis and subsequent formation of nuclei resulted in a contraction and densification of original crystal structure, with formation of lamellar sheets as observed in TEM micrographs. The resulting anatase nanoparticles had a large surface area of 288 m2 g−1 [54].
The very same phenomenon was observed for a tetranuclear titanium alkoxide complex modified with tert-butylphosphonic acid and acetylacetonate ligands (Fig. 2) [46]. Hence, thermal hydrolysis of bimetallic single-crystal oxo-alkoxide complexes may provide a route to highly porous complex oxides, which remains to be explored.
Formation of anatase nuclei from a single crystal. A single crystal (a) hydrolyses in contact with water (b) which leads to the formation of lamellar sheets upon contraction and densification, which on further hydrolysis results in formation of anatase nuclei (c). d TEM micrograph of lamellas, and (e) HRTEM micrograph of anatase nuclei forming upon hydrolysis from a crystal fragment of [(Ti4(iOPr)4(acac)4(tBuPO3)4] (acac = acetylacetonate) after contact with water. Adapted from ref. [46] with permission from the Royal Society of Chemistry
The first step in the formation of the oxide phase is the generation of Ti-O-Ti bonds [55], thus, oxo-alkoxides can be considered intermediates for oxide materials from the sol-gel process [56]. Hence, the incorporation of a heterometal, M, to form Ti-O-M, in TOAs could provide a route to obtain both doped titania and complex titanates. However, if the ionic radius and/or charge of the dopant are too different from Ti4+, separate oxide phases can form during hydrolysis/thermolysis of heterometallic complexes [48]. Doeuff et al. [57] used electron paramagnetic resonance (EPR) to study the incorporation of Cr3+ into titania, starting from titanium(IV) butoxide and chromium(III) acetylacetonate. Upon heating of the two precursors, several bimetallic intermediates could be distinguished. After hydrolyzing and annealing of the intermediates, EPR indicated both volume and surface doping of Cr3+ in the anatase. A recent study on rare-earth element-doped titania employing photoluminescence spectroscopy suggested the dopants of larger ionic radii (compared to Ti4+) can be incorporated in low-crystallinity anatase lattice. However, as the annealing temperature was increased and the crystallinity improved, the dopants appeared to migrate out towards the anatase surface [22].
3 First-row transition metals
Of the transition metals, the first-row elements dominate as dopants for TOAs, and in particular Fe, Mn, Co, and Zn. As the nuclearity increases, they tend to adapt a spherical shape and based on the position of the heterometal, two sub-classes of the spherical structures can be distinguished; peripherally and internally doped TOAs. In the peripherally doped TOAs the dopant is located on the surface and in the internally doped TOAs the dopant is encapsulated within the titanium-oxygen framework. Complexes containing simultaneously internal and external dopants also exists. It can also be noted that most TOAs containing two or more transition metal dopants commonly contain an additional chelating/bridging ligand attached to the dopant. Examples of synthetic strategies are given in Scheme 1.
3.1 Vanadium
A few titanium-containing polyoxovanadate alkoxy complexes have recently been reported by Matson’s group (Fig. 3) [58, 59]. The Lindqvist type [(TiOCH)3V5O6(OCH3)12)]− was formed by reaction between [Ti(OMe)4]4 and VO(OCH3)3 in methanol at elevated temperature with [NBu4][BH4] as reductant. They latter extended this work to incorporate a second titanium ion, to obtain [(TiOCH3)2V4O5(OCH3)12] by increasing the Ti equivalents and removing the reductant [59]. The substitution of a V for a Ti4+ ion demonstrated increased oxidation potential for the vanadyl complex [58].
3.2 Chromium
A small chromium-containing complex, [Ti3O{CrCl}(OEt)14], was reported by Eslava and co-workers [60] from solvothermal reaction between titanium(IV) ethoxide and chromium(II) chloride. The chromium ion connects to the (Ti3O) fragment via three µ3-OR bridges and a µ4-oxygen bridge. It had a rather weak absorption in the visible region. Another, more peculiar, chromium-containing TOA was produced by Yi et al. [61]. They reacted an aqueous solution of titanyl sulfate (TiOSO4) with Na[CpCo(P(O)-(OEt)2)3] (Cp = cyclopentadiene) and Na2Cr2O7 to obtain, via recrystallization from a dichloromethane: hexane mixture, the trimetallic complex [TiCpCo(P(O)-(OEt)2)3])2(μ-CrO4)3]. The two Ti4+ ions are bridged via three tetrahedral CrO4-ligands and are further bridged to the CoCp unit via three diethyl phosphate molecules. It could catalyze the oxidation of benzyl alcohol to benzaldehyde. Zhang and co-workers [62] reported a larger TOA with high content of chromium in from solvothermal reaction between titanium(IV) isobutoxide with Cr(CH3COO)3 (approx. 5:4 ratio) and benzoic acid (HBA) in acetonitrile. A ring-like complex formulated as [Ti12O18Cr6(BA)30] was obtained. Altering {TiO6} and (CrO6) octahedra were linked by µ2-benzoate and oxo-ligands. The complex displayed an improved visible absorption with a maximum around 610 nm, and the absorption continued into the near infrared region. It was reported to have a hydrogen production rate of 147.86 μmol·g−1·h−1 under simulated solar light. PXRD characterization before and after catalysis suggested operational stability of the electrodes. Examples of Cr-TOAs are shown in Fig. 4.
Structures of selected Cr-TOAs. a [Ti3O{CrCl}(OEt)14] and (b) [TiCpCo(P(O)-(OEt)2)3])2(μ-CrO4)3]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Light blue is chromium, green is titanium, red is oxygen, dark green is chloride, orange is phosphorous, dark blue is cobalt, and grey is carbon
3.3 Manganese
Manganese is one of the most common TM dopants for TOAs and structures representing nuclearities from Ti1 to Ti28 and containing from one to four manganese ions have been reported, with particular contribution from the groups of Wright and Coppens.
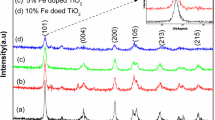
One early structure was synthesized by Sobota’s group [63] by reaction between [Ti(iOPr)4] and 0.5 eq. [Mn4(µ2-Cl)Cl3(OMe)4(2-ME)3]2 (2-ME = 2-methoxyethanol) at room temperature which afforded the complex [TiMn3(μ3-2-ME)3)2(μ2-2-ME)3)3(μ-Cl)Cl2(iOPr)2]. While it was not intended as a SSP, they investigated the complex as a catalyst for ethylene polymerization with AlEt2Cl and methylaluminoxane for which it showed some activity. Eslava et al. [64] reacted [Ti(OEt)4]4 with MnCl2 in a solvothermal route with ethanol as solvent to obtain [Ti4O{Mn2Cl3}(OEt)15]. Several [Ti4O{MCl}(OEt)15)] complexes were obtained from the same route (and equivalents) where M = Co, Zn, Fe, and Cu. For Mn, two manganese ions were incorporated into the structure, where the two MnII were connected via a µ-Cl bridge, and connected to Ti4+ centers via bridging ethoxide groups. Unlike the Co, Fe, and Cu analogues, the manganese complex did not absorb in the visible spectrum. Li and co-workers [65] reported a relatively big TOA [Ti18O30Mn{MnPhen}3(OEt)20] containing four Mn2+ ions. This compound was produced via solvothermal treatment of [Ti(OEt)4]4 and manganese acetate dihydrate with 1,10-phenanthroline as additive in ethanol as solvent. Following calcination in air at 600 °C it yielded manganese-doped titania, predominantly in the rutile phase with some segregated α-Mn2O3 (Fig. 5).
PXRD patterns of [Ti18O30Mn{MnPhen}3(OEt)20] annealed at different temperatures. A is anatase, R is rutile, and # is α-Mn2O3. Reproduced from ref. [65] with permission from the Royal Society of Chemistry
Coppens’ group [66] reported another Mn-TOA, [Ti11O14{MnX}(OiPr)17] via reaction between [Ti(iOPr)4] and MnX2 (X = Cl, Br, or I) in a solvothermal method. The inclusion of a peripheral Mn2+ ion to TOA resulted in some red-shift of the UV-vis absorption spectrum but considerably less compared to the FeII analogue. Yang and colleagues [67] also utilized a solvothermal method to synthesize [Ti12O10Mn2(Cat)2(BA)4(OEt)22] from [Ti(iOPr)4] and Mn(CH3COO)2 with catechol (Cat) and benzoic acid (BA) as stabilizing ligands. The manganese ions were integrated into the inorganic oxo-core via µ2-O, µ3-O, benzoate, and catecholate bridges. Electrodes of the complex prepared by dip-coated indium tin oxide (ITO) substrates had an improved photocurrent response compared to their reference titania, but it was substantially lower compared to the cobalt analogue.
Chen et al. [68] used a solvothermal reaction between titanium(IV) ethoxide and manganese(III) acetate, with ammonium bromide as additive, to obtain a large, Keggin-like H2[Ti28O38Mn(OEt)40] complex with a tetrahedral manganese center. Notable, MnIII was reduced to MnII in the structure, but the complex could not be obtained by using manganese(II) acetate as reagent. The band gap of the Ti28Mn complex (2.74 eV) was substantially lower compared to undoped Ti28 complex (3.43 eV), as determined by diffuse-reflectance spectroscopy. In another publication, Lv and co-workers [69] investigated the effect on the band gap by doping a Ti14 complex with Mn2+, [Ti14O14Mn(OEt)28(OH)2], as it previously had been reported to have a significantly lowered band gap. They did, however, found the actual band gap to be higher than that of anatase-TiO2, ca. 3.36 eV. For the Fe2+ and Gd2+ analogues, band gaps were determined to 3.22 eV and 3.57 eV respectively, by UV-vis diffuse reflectance spectroscopy. The previously reported 2.65 eV band gap was proposed to be caused by contamination of amorphous Mn-doped titania. Chen et al. [70] reported another Mn-TOA formulated as [Ti13O16Mn4(THME)4Br4(OEt)12] obtained via solvothermal reaction between [Ti(OEt)4]4 and MnBr2 in ethanol with 1,1,1-tris(hydroxymethyl)ethane (THME). It consists of a semi-spherical oxo-core of octahedrally coordinated Ti4+ ions, caped with terminal ethoxide ligands and four ƞ3-THME ligands. Four peripheral, octahedrally coordinated manganese ions are present. They form a polymeric network via Mn(µ-Br)2Mn bridges between the individual complexes. Diffuse reflectance spectroscopy determined the band gap to ca. 2.57 eV. Zhang and co-workers [71] synthesized a smaller complex, [Ti6O4Mn2(PPA)2(Sal)6(OEt)4(EtOH)2], from reaction between [Ti(OEt)4]4 and an unspecified manganese salt with phenylphosphonic acid (PPA) and salicylic acid (Sal) as additives. It contains two (Ti3O) sub-units liked by two µ2-oxygen and two bridging phenylphosphonate ligands to form a cube-like structure. The PPA ligands acts as bridges connecting the titanium ions while the Sal ligands act both bridging and chelating. Each titanium center has one chelating Sal ligand, which are positioned to form an “ion trap” that can coordinate a metal ion with up to six oxygen bonds. The Mn2+ ion utilizes five of these bonds. Dissolution of this complex in a Triton X-100 solution led to the self-assembly of flower-like nanosheets that appeared to be amorphous from HRTEM imaging (Fig. 6). Photocatalytic degradation of rhodamine B by the flower-like assemblies was investigated and reported to be about 80% after 30 min after exposure to 280 W xenon lamp. This was considerably higher compared to the zinc and terbium analogues of ca. 25% and 18%, respectively. Examples of Mn-TOAs are given in Fig. 7.
Self-assembled structures from a Mn-TOA. a SEM micrograph of flower-like assemblies of [Ti6O4Mn2(PPA)2(Sal)6(OEt)4(EtOH)2] dissolved in Triton X-100. b HRTEM micrograph indicating an amorphous structure. Reproduced from ref. [71] with permission from the Royal Society of Chemistry
Structures of selected Mn-TOAs. a [Ti4O{Mn2Cl3}(OEt)15]. b [Ti12O10Mn2(Cat)2(BA)4(OEt)22]. c [Ti18O30Mn{MnPhen}3(OEt)20]. d H2[Ti28O38Mn(OEt)40]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Purple is manganese, green is titanium, red is oxygen, dark purple is nitrogen, dark green is chloride, and grey is carbon
3.4 Iron
Iron represents another popular dopant for TOAs which may stem from the improved photocatalytic performance of iron-modified titania as an effect of red-shifted absorption spectra and improved charge carrier-transport [72, 73]. The first iron-containing complexes, [Ti2{FeCl}(iOPr)9] and [Ti3(OMe)2(iOPr)9][TiO{FeCl}4(iOPr)9], were reported by Nunes and co-workers [74] in 2002 by reaction between [Ti(iOPr)4] and FeCl2 in a toluene: isopropanol mixture in the presence of KiOPr. In a follow-up work, the same group investigated the decomposition products of [Ti2{FeCl}(iOPr)9] under various conditions. PXRD indicated complex mixtures of different iron and titanium oxide phases in all cases [75]. Three additional Fe-TOAs, [Ti11O14{FeCl}(iOPr)17], [Ti11O14{FeBr}(iOPr)17], and [Ti11O14{FeI}(iOPr)17], analogues to [Ti12O16(iOPr)16], were latter prepared by Coppens’ group [66]. [Ti(iOPr)4] was reacted with FeCl2, FeBr2, or Fe(CH3COO)2 + NH4I in presence of salicylic acid and heated in a sealed glass tube at 150 °C. The three compounds are isostructural, varying only in the anion coordinating to iron center, however, no salicylate ligands were incorporated into the complexes. The three FeTi11 complexes demonstrated improved absorptions in the visible region, with little apparent influence from the halogen anion.
Eslava and colleagues [64] prepared a small Fe-TOA from a solvothermal reaction between [Ti(OEt)4]4 and FeCl2 which afforded [Ti4O{FeCl}(OEt)15]. The Fe2+ ion connected to the TOA via three bridging ethoxide groups. The complex had a red-shifted absorbance into the visible region up to ca. 600 nm. The same group latter utilized a post-functionalization route to modify [Ti7O4(OEt)20] with FeCl2 in toluene, which afforded [Ti7O5{FeCl}(OEt)18Cl)] [60]. The Fe2+ ion is connected via three bridging ethoxide groups. One Ti4+ ion located three bonds away from the iron dopant had a terminal ethoxide ligand replaced by a chloride ion. An increased absorbance of visible light was observed for the iron-containing complex in UV-vis measurements. A large, peripheral type Fe-TOA complex, [Ti17O28{FeIIPhen}2(iOPr)16] was reported by Jarzembska et al. [76] by solvothermal reaction of [Ti(iOPr)4] and Fe(CH3COO)2 with 1,10-phenanthroline (Phen) as additive in isopropanol. The structure consisted of a spherical (Ti17O28(iOPr)16) core with two Fe2+ ions in “para-position”, connected via µ2-O and µ3-O bridges. Each Fe2+ center contained one chelating Phen ligand. Exposure of visible light to a thin film of the complex deposited on a fluorine doped tin oxide (FTO) electrode generated a photocurrent between 400 nm and 640 nm. Lv and colleagues [69] synthesized a Keggin-like [Ti14O14Fe(OEt)28(OH)2] cage compound with the FeII ion encapsulated in the structure. The iron ion is connected to Ti4+ solely via µ3-O bridges in a pseudotetrahedral arrangement. Given the relatively high condensation number (Ti/O = 1), the structure hydrolyzed only slowly at ambient conditions. The band gap was determined to 3.22 eV by diffuse reflectance spectroscopy. A selection of Fe-TOAs is shown in Fig. 8.
Structures of selected Fe-TOAs. a [Ti2{FeCl}(iOPr)9]. b [Ti17O28{FeIIPhen}2(iOPr)16]. c [Ti7O5{FeCl}(OEt)18Cl)]. d [Ti14O14Fe(OEt)28(OH)2]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Orange is iron, green is titanium, red is oxygen, dark green is chloride, purple is nitrogen, and grey is carbon
3.5 Cobalt
An early Co-TOA was reported by Eslava and co-workers [77] when they obtained the complex [Ti7O5{CoCl}(OEt)19] when attempting to synthesize [Ti2{CoCl}(OEt)9] from a 1:1 reflux of [KTi2(OEt)9] and CoCl2 in toluene. In an attempt to obtain the complex in better yield they solvothermally reacted [Ti(OEt)4]4 with CoCl2 in a 7:1 ratio but instead obtained [Ti4O{CoCl}(OEt)15] as an apparently favorable product since it was afforded over a wide range of synthetic conditions. In a follow up work, the same group successfully synthesized [Ti7O5{CoCl}(OEt)19] via post-functionalization of [Ti7O4(OEt)20] with 1 eq. of CoCl2 in toluene [60]. It had a slightly increased visible absorption in the 500–650 nm range. Lai and co-workers [78, 79] reported two use of two additional Co-doped TOAs, [Ti12O15(iOPr)17][Ti15O24{CoBr}6(iOPr)18Br] and [Ti11O14{CoI}(iOPr)17] as SSPs for production of CoTi materials for (photo)electrochemical water oxidation. They were prepared by solvothermal reaction between [Ti(iOPr)4] and CoX2 (X = Br or I) in 9:1 ratio. The complexes were dissolved in dichloromethane and drop-casted on FTO-coated glass. The exposure of atmospheric water caused hydrolysis of the complexes which resulted in the entrapment of the cobalt ions in an amorphous titania network (Fig. 9). The authors reported an oxygen production of ca 9.4 µmol h−1 and a Faradaic efficiency of 90% from the electrode prepared from [Ti12O15(iOPr)17][Ti15O24{CoBr}6(iOPr)18Br]. Based on similar results for the electrode prepared from [Ti11O14{CoI}(iOPr)17], they concluded that very similar materials were formed during the hydrolysis of the two SSPs.
Electrode materials from hydrolyzed Co-TOAs. SEM micrographs of [Ti12O15(iOPr)17][Ti15O24{CoBr}6(iOPr)18Br] (a) and [Ti11O14{CoI}(iOPr)17] (b) deposited on FTO. Reproduced from ref. [78] with permission from the Royal Society of Chemistry
Wang et al. [80] reported the doubly doped Co-TOA [Ti4O4Co2(BA)12(CH3CN)2] obtained by reaction between [Ti(iOPr)4] and Co(acac)2 in acetonitrile with benzoic acid (BA) as additive. This compound displayed good absorption over the entire visible spectrum. Density functional theory (DFT) calculations by the authors indicated that a metal-to-core charge transfer from the cobalt dopant contributed to the increased absorption of visible light. Photoelectrodes were prepared by spin-coating ITO glass electrodes with a dichloromethane solution of the complex. A stable photocurrent of 0.9 µA cm−2 was reported. Another larger, doubly doped complex, [Ti12O10Co2(Cat)2(BA)4(OEt)22] was prepared from the reaction between [Ti(iOPr)4] and Co(CH3COO)2 in anhydrous ethanol with benzoic acid (HBA) and catechol (H2CA) as additives. An electrode was prepared by dip-coating a TiO2/ITO substrate in a dichloromethane solution of the complex. X-ray photoelectron spectroscopy analysis of the electrode indicated some partial hydrolysis, based on the shift of the O1S signal as compared to TiO2. A stable photocurrent response with a current density of 12.0 µA cm−2 was reported [67].
A CoTiO3 SSP was prepared by Ehsan and co-workers [24] by reacting Co(CH3COO)2 and [Ti(iOPr)4] (in a 1:2 ratio) in tetrahydrofuran (THF) with excess of trifluoroacetic acid (TFA). This afforded [Ti4O6Co2(TFA)8(THF)6]. The complex was dissolved in ethanol and aerosol-assisted CVD on FTO glass with subsequent annealing was employed to produce electrodes. During annealing (500 –600 °C) the complex decomposed into a CoTiO3:TiO2 composite, consisting of pure ilmenite and rutile phases for CoTiO3 and TiO2, respectively (Fig. 10). This composite was evaluated for electrochemical detection of dopamine, and a limit of detection of 0.083 µM was reported. A selection of Cr-TOAs is shown in Fig. 11.
Production of a CoTiO3-TiO2 thin film from a Co-TOA SSP. a XRD patterns from the deposition of [Ti4O6Co2(TFA)8(THF)6] on FTO glass at different temperatures and (b) SEM cross-section of CoTiO3-TiO2 composite obtained at 600 °C. Reproduced from ref. [24] with permission from the Royal Society of Chemistry
Structures of selected Co-TOAs. a [Ti4O{CoCl}(OEt)15]. b [Ti7O5{CoCl}(OEt)19]. c [Ti20O28{Co2I3}(HPO3)(OEt)23]. d [Ti12O10Co2(Cat)2(BA)4(OEt)22]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Blue is cobalt, green is titanium, purple is iodine, dark green is chloride, and grey is carbon
A series of cobalt-doped TOAs were prepared by Lv et al. [81] as models for Co-doped titania; [Ti4O{CoBr}(OEt)15], [Ti7O5{CoBr}(OEt)19], [Ti24O34{CoBr}2(OEt)30], and [Ti20O28{Co2I3}(HPO3)(OEt)23]. They were synthesized by solvothermal reaction between [Ti(OEt)4]4 and CoBr2 or CoI2. The two larger TOAs, [Ti24O34{CoBr}2(OEt)30] and [Ti20O28{Co2I3}(HPO3)(OEt)23], displayed more red-shifted absorption edges compared to the two smaller TOAs (likely the quantum confinement effect), and absorbed strongly between 600 nm and 750 nm. The two smaller TOAs, [Ti4O{CoBr}(OEt)15] and [Ti7O5{CoBr}(OEt)19], on the other hand, displayed more blue-shifted absorption edge and had very similar absorption spectra to their previously reported [Ti4O{CoCl}(OEt)15]. From DFT calculations of the complexes, they suggested a dipole-induced decrease in band gap caused by the inclusion of the Co2+ dopant. This conclusion was challenged in a latter publication by Coppens [66].
3.6 Nickel
Kessler and co-workers [34] prepared a small Ni-doped TOA, [Ti2Ni2(OEt)8(acac)4], by refluxing Ni(acac)2 and [Ti(OEt)4]4 in toluene with subsequent crystallization at room temperature. Microhydrolysis of [Ti2Ni2(acac)4(OEt)8] by addition of 99.5% ethanol resulted in formation of a new complex of very different stoichiometry, [TiONi5(acac)6(OEt)6] [82]. The [Ti2Ni2(acac)4(OEt)8] complex was latter used by Tahir et al. [83] as a SSP for producing NiTiO3 by thermal decomposition at 500 °C (Fig. 12). Another small Ni-TOA was prepared by Eslava et al. [64] from reaction of [Ti(OEt)4]4 with 0.06 eq. NiCl2 ethanol and afforded after recrystallization in toluene the compound [Ti2{NiCl}(OEt)9]2, which consists of two triangular {Ti2Ni} units linked via µ2-OEt and µ3-OEt bridges, and the {Ti2Ni} units are in turn linked via two µ2-Cl bridges. UV-vis measurement in solution (toluene) revealed a strongly blue-shifted absorption edge ascribed to the lack of a titanium-oxo core, but with two small absorption bands in the visible region. A selection of Ni-TOAs is shown in Fig. 13.
Production of a NiTiO3 thin film from a Ni-TOA SSP. a SEM micrograph NiTiO3 films from AACVD, deposited on ITO glass. b PXRD of NiTiO3 deposited on glass substrate. Reproduced from ref. [83] with permission from the Royal Society of Chemistry
Structures of selected Ni-TOAs. a [Ti2Ni2(acac)4(OEt)8]. b [Ti2{NiCl}(OEt)9]2, c [Ti12O10Ni2(Cat)2(BA)4(OEt)22]. d [Ti11O14{NiBr}(iOPr)17]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Violet is nickel, green is titanium, red is oxygen, dark green is chloride, dark red is bromide, and grey is carbon
Hu et al. [84] reacted [Ti(iOPr)4] with low equivalents of NiX2 (X = Cl, Br, or I) to obtain a series of isostructural Ni-doped TOA, [Ti11O14{NiX}(iOPr)17], differing only in the halide anion. The peripheral Ni2+ is connected to the TOA via three µ3-oxygen bridges, and has one terminal halogen ligand. The authors performed extensive investigation of the photo-responses of the compounds. The inclusion of Ni2+ did extend absorption of the complex into the visible region, as compared to the Ti11 and Ti12 references. Interestingly, a dependence on the halide anion was observed where the absorption edge was red-shifted most in the order Cl > Br > I. No photocurrent could be observed from the complexes under visible light irradiation. It was concluded that the doping with Ni2+ (and the Zn2+ analogue) had a negative impact on the UV-photoactivity.
A linear-type complex, [Ti12O10Ni2(Cat)2(BA)4(OEt)22] were recently reported by reaction between [Ti(OEt)4]4 and Ni(CH3COO)2 in anhydrous ethanol with two different additives; benzoic acid (HBA) and catechol (H2Cat) [67]. It features two central Ni2+ ions connected via edge-sharing, and µ3-oxygen bridges. The Ni2+ are further connected to the (Ti6) units via bridging catecholate, benzoate, and µ3-oxygen bridges. The photocurrent density of the complex, 1.8 µA cm−2, were smaller compared to that of the Mn and Co analogues, 12.0 and 3.6 µA cm−2, and only moderately higher compared their reference complex, [Ti6O4(Cat)2(BA)2(iOPr)10], of 0.8 µA cm−2 indicating influence from the TM dopant. However, the photocurrent measurements were conducted in an aqueous Na2SO4 solution and no characterization of the electrodes after reaction was reported. A rare example of two co-crystallized Ni-doped TOAs [Ti8O10Ni4(acac)4(C6H5COO)16(CH3CN)2] + [Ti4O4Ni2(acac)2(C6H5COO)10] ([Ti4Ni2 + Ti8Ni4]) were recently reported by Wang and colleagues [85]. They were obtained by solvothermal reaction between [Ti(iOPr)4] and Ni(acac)2 (3:1 Ti:Ni ratio) in acetonitrile with benzoic acid as additive. At 100 °C, the [Ti4Ni2] unit was obtained, and by increasing the temperature to 120 °C, the [Ti8Ni4 + Ti4Ni2] unit was instead afforded. The [Ti8Ni4 + Ti4Ni2] complex exhibited an improved absorption in the visible region, which was ascribed by the authors to be caused by [Ti4Ni2]-to-[Ti8Ni4] charge-transfer. Photocurrent responses under a 300 W xenon lamp were determined to ca. 0.6 μA cm−2 and ca. 0.8 μA cm−2 for [Ti4Ni2] and [Ti8Ni4 + Ti4Ni2], respectively. PXRD measurements before and after reaction were claimed to indicate stability under the working conditions.
3.7 Copper
An early Cu-TOA was reported by Veith and co-workers [86] by a simple metathesis reaction between [KTi2(iOPr)9] and CuCl2 to afford [Ti2{CuCl}(iOPr)9]. It appeared monomeric in solution from 1H and 13C NMR analyses. Later, Hamid and colleagues [87] attempted to synthesize a Cu-TOA SSP for producing copper titanate films. By reacting Ti(DMAE)4 (DMAE = N,N-dimethylaminoethanolate) and Cu(CH3COO)2 dihydrate in 2:3 ratio using toluene as solvent the compound [Ti4O6Cu6(DMAE)6(OAc)9(OH)] was obtained. Thermal decomposition at 300 °C resulted in a ca. 0.77:0.23 mixture of Cu3TiO4 and TiO2. Eslava and co-workers [64] prepared a small Cu-doped TOA, [Ti4O{CuCl}(OEt)15] as a potential SSP. While the production of Cu-doped TiO2 from it was not attempted, UV-vis measurements displayed substantially improved absorption in 550 nm to 800 nm region. Liu et al. [88] prepared a novel complex [Ti6O2Cu2(FSA)6(CH3COO)2(CH3CN)2(iOPr)10] by solvothermal reaction between [Ti(iOPr)4], and Cu(CH3COO)2 in a 3:1 mixture with 3-fluorosalicylic acid (FSA) as additive and acetonitrile as solvent. The obtained complex consisted of two typical (Ti3O) units, separated by two (CuIIO6) octahedra. Rather extensive photochemical studies were conducted on the compound and their reference complex, [Ti7O4(FSA)4(iOPr)12]. The measurements were, however, conducted in aqueous media and no characterization of their compounds after measurements in water were reported. This is very important considering the hydrolytic instability of titanium oxo-alkoxides (even for the ligand-modified ones) [22, 34, 46]. Two additional Cu-TOAs with the general formula [Ti5O6Cu4(OOCR)16] were reported by Artner and co-workers [89] from reaction between [Ti(iOPr)4] and Cu(CH3COO)2 with either methacrylic acid (MA) or propionic acid (PA), in a Ti:Cu ratios 1:2 and 1:1 for MA and PA, respectively. However, in both cases complexes with Ti:Cu ratios of 5:4 were obtained. No application was reported. A similar compound to the ones reported by Artner were synthesized by Wang et al. [80], but methacrylic acid and propionic acid were replaced with benzoic acid. Their complex, [Ti5O6Cu4(C6H5COO)16], was deposited on ITO glass from a dichloromethane solution, and photocurrent and hydrogen production under a 300 W xenon lamp were investigated. A stable photocurrent of ca. 0.8 µA cm−2 and a hydrogen production of 42.02 µmol g−1 h−1 were reported. In agreement with Eslava and colleagues [64], absorption in the 550–800 nm range was improved. The authors concluded the complex was stable under operational conditions based on FTIR and PXRD analyses. A selection of Cu-TOAs is shown in Fig. 14.
3.8 Zinc
Hu and co-workers [84] synthesized a [Ti11O14{ZnX}(iOPr)17] (X = Cl or Br), a peripheral type complex, as part of an isostructural series of metal-doped TOAs. Photocurrent activity turned out to be lower compared to their undoped [Ti12O16(OiPr)16] and [Ti11O13(iOPr)18] references. Eslava et al. [64] reported a low-nuclear [Ti4O(ZnCl)(OEt)15] complex. UV-vis measurements in solution displayed no visible light absorbance and a very blue-shifted UV-absorption.
The structure [TiZn(2-ME)(THF)Cp2Cl2] (2-ME = 2-methoxyethanol) was produced by reacting [TiCp2Cl2] with metallic Zn (1:2 Ti:Zn ratio) in a mixture of THF and 2-methoxyethanol at room temperature [90]. The two chloride ions from the titanocene dichloride complex were replaced by a chelating 2-methoxyethanol ligand using the excess Zn(OR)2 to extract the chloride ions to form Zn(OR)Cl and/or ZnCl2 [91]. The compound was systematically investigated as a SSP for zinc titanates by thermolysis. After thermal decomposition of the compound by annealing at 800 °C, a mixture of ZnTiO3, rutile-TiO2, anatase-TiO2, Zn2Ti3O8, and ZnO were detected by PXRD. At 900 °C, rutile-TiO2 and Zn2TiO4 phases seemed to dominate [90]. Artner and co-workers [92] prepared a Zn-TOA [Ti4O4Zn2(OMc)10(iOPr)2] (OMc = methacrylic acid) from a room-temperature reaction with stochiometric amounts of [Ti(iOPr)4] and Zn(CH3COO)2 with methacrylic acid as additive with a small amount of dichloromethane as solvent. The complex is structurally related to the TOA [Ti6O4(OR)8(OOCR´)8], forming two {Ti2Zn} subunits sharing a µ3-oxygen bridge and being connected via bridging methacrylate molecules and µ2-oxygen bridges. Two binuclear structures, [TiZnEt(H3DPTA)(iOPr)2] and [TiZnPhen(H3DPTA)(iOPr)2] (H6DPTA = diphenolate tetraamine) were prepared by reaction between [Ti(iOPr)4] and diethyl zinc or diphenyl zinc in toluene at room temperature. These two complexes were employed as catalysts in various reactions and had moderate activity for ring opening polymerization of ε-caprolactone [93].
A bimetallic zinc-titanium complex for production of ZnTiO3 via aerosol assisted CVD was reported by Ehsan and co-workers [94]. By reaction [Ti(iOPr)4] with Zn(CH3COO)2 in a 2:1 ratio with an excess of trifluoroacetic acid (TFA) in THF they obtained [Ti4O6Zn2(TFA)8(THF)6]. It consists of a Ti4-oxo core with two octahedral zinc ions located in “para-position” connected via µ3-oxygen bridges and bridging TFA ligands. By employing an aerosol-assisted CVD method, they produced a mixed TiO2:ZnTiO3 film (Fig. 15). The phase-composition of the film was dependent on the solvent used and was most efficient for an acetonitrile solution of the complex which gave a film consisting of 85% ZnTiO3, 12% rutile-TiO2, and 3% anatase TiO2. A selection of Zn-TOAs are shown in Fig. 16.
Production of a ZnTiO3-TiO2 thin film from a Zn-TOA SSP. a PXRD of films from [Ti4O6Zn2(TFA)8(THF)6] deposited from different solvents and (b) SEM micrograph of a cross-section of ZnTiO3-TiO2 composite film deposited from an acetonitrile solution of the complex. Reproduced from ref. [94] with permission from the Royal Society of Chemistry
4 Second-row transition metals
With the exception of zirconium, much fewer TOAs doped with second-row transition metals have been reported to date. Some of the most relevant examples are summarized below. Contrary to the first-row transition metals, here are more examples of compounds with higher number of dopants, e.g., Ti:TM ratios 2:1, 1:1, and 1:2. The application of additional bridging heteroligands also appears important for these structures. Examples of synthetic strategies are given in Scheme 2.
4.1 Yttrium
The first Y-TOA, [Ti2Y(iOPr)9Cl2], was reported by Veith and colleagues in 1997 [86] via a metathesis reaction between [KTi2(iOPr)9] with YCl3 in a heated iPrOH: toluene mixture. The yttrium ion is connected via doubly and triply bridging isopropoxide ligands. No applications were reported. An analogous structure, [Ti2Y(iOPr)9(NO3)2], was recently reported from a solvothermal reaction between [Ti(iOPr)4] and Y(NO3)3•6H2O in toluene [22]. It was investigated as a potential SSP for yttrium titanate or yttrium-doped titania via hydrolysis. However, anatase-phase titania was the only observed product (Fig. 17). Three additional Y-TOAs were prepared Schubert’s group [95] as part of their investigation of methacrylate-substituted titanium oxo-complexes as precursors for hybrid materials. Despite varying the [Ti(nOPr)4]:Y(OCH3)3 ratio from 2:1 to 18:1, three complexes with the common core {Ti4Y2O4} were obtained, differing only in the composition of coordinating ligands, i.e., methoxide (MeOH), methacrylic acid (McOH), and 2-methoxymethanol (2-ME, the solvent), yielding [Ti4O4Y2(OMc)14(2-ME)2], [Ti4O4Y2(OMc)14(McOH)2], and [Ti4O4Y2(OMc)12(2-ME)2(McOH)2]. Examples of Y-TOAs are given in Fig. 18.
HRTEM micrograph of [Ti2Y(iOPr)9(NO3)2] hydrolyzed in water, circles indicate anatase crystallites. The lattice fringe distance in the insert is ca. 0.2 nm, corresponding to the 2 0 0 plane of anatase. Reprinted with permission from ref. [22]. Copyright 2021 American Chemical Society
4.2 Zirconium
There has been a certain interest in heterometallic Zr-Ti complexes as SSPs for zirconium titanates. The first Zr-TOAs were prepared by Caulton and co-workers [96] in a facile reaction between a zirconium pinacolate (Pin) complex [Zr2(Pin)2(HPin)4] and [Ti(iOPr)4] in THF. By using either Zr:Ti ratios 1:1 or 1:2, [TiZr2(Pin)4(HPin)2(iOPr)2] or [Ti2Zr2(Pin)6(iOPr)4], respectively, were obtained. In both structures Ti is pentacoordinated and Zr and Ti are connected via bridging pinacolate ligands. Their initial attempts to produce Zr-TOA from mixtures of homoleptic zirconium and titanium alkoxides were apparently unsuccessful. However, the introduction of chelating and bridging functions from the pinacolate ligands, as well as reduced steric hindrance, was proposed to facilitate the formation of the two Ti-Zr complexes.
Several structures were reported by Kemmitt’s group in the early 2000s [97, 98]. [Ti2Zr2(C3H7O)12(MDEA)2] was produced by reacting stochiometric amounts of [Ti(iOPr)4] and Zr(nOPr)4 with N-methyldiethanolamine (H2MDEA) in benzene. They latter prepared the dinuclear [TiZr(MDEA)3(C3H7O)2] simply by doubling the amount of H2MDEA. Moraru et al. [99] reported four additional Zr-TOAs; [Ti4O6Zr4(OMc)16(OBu)4], [Ti2O4Zr4(OMc)14(OBu)2], [Ti4O4Zr2(OMc)10(OBu)6], and [Ti2O6Zr6(OMc)20] by reaction between [Ti(OBu)4], [Zr(OBu)4] or [Zr(OPr)4] with excess methacrylic acid in butanol. The starting Ti:Zr ratios were well reflected in the structures, except for the Ti2Zr6 complex, which co-crystallized with the Ti4Zr2 complex. These compounds feature (ZrO8) units linked in a zigzag pattern and terminated by edge- or corner-sharing (TiO6) octahedra. A latter study from the same authors investigated the core-stability after functionalization with a variety of organic ligands to produce different hybrid materials, exploiting the alkene function in methacrylic acid. According the extended absorption fine structure (EXAFS) analysis, the metal-oxo frameworks were retained after functionalization [100].
Spijksma and co-workers prepared the SSP [(Ti(iOPr)3)2Zr(DEA)3] (DEA = diethanol amine) by a reaction 1:2:3 of [Zr(iOPr)4], [Ti(iOPr)4], and DEA, where the two titanium centers where connected to the nonacoordinated Zr4+ via bridging DEA ligands [101]. In a subsequent publication, this complex was successfully utilized as a SSP for production of essentially pure microporous srilankite (ZrTi2O6) (Fig. 19) by thermal decomposition at 800 °C, much lower compared to the traditional solid-state reaction [26]. A selection of Zr-TOAs is given in Fig. 20.
Composite membrane from a Zr-TOA. a SEM micrograph of ZrTi2O6 film deposited on a γ-Al2O3 substrate and (b) PXRD of [(Ti(iOPr)3)2Zr(DEA)3] treated at different temperatures. The strongest diffraction planes for srilankite are indicated by an asterisk. Copyright (2006) Wiley. Used with permission from ref. [26]
Structures of selected Zr-TOAs. a [(Ti(iOPr)3)2(Zr(DEA)2)3]. b [TiZr(MDEA)3(C3H7O)2]. c [Ti4O6Zr4(OMc)16(OBu)4]. d [Ti2O4Zr4(OMc)14(OBu)2]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Light blue is zirconium, green is titanium, red is oxygen, blue is nitrogen, and grey is carbon
4.3 Niobium
Nb-TOAs are sparse, but one example was recently reported by Yuan and co-workers [102] from the reaction between [Ti(iOPr)4] and NbCl5 in n-propanol with embonic acid (EA) as additive. This afforded the structure H4[Ti2O2Nb2(EA)4(nOPr)2]. The titanium and niobium ions are connected by µ2-oxygen bridges and embonate ligands. The niobium ions further have one terminal n-propoxide ligand each. A band gap of ca. 1.4 eV was reported.
4.4 Molybdenum
A Mo-TOAs of the Linqvist-type structure, TBA3[TiO18Mo5(iPrO)], was prepared by Errington and colleagues [103] from the reaction between (TBA)2[Mo2O7], (TBA)4[Mo8O26], and [Ti(iOPr)4] in acetonitrile (TBA = tetrabutylammonium) followed by the addition of water (1.5 eq to Ti) and subsequent heating to 100 °C. In a latter publication the same authors [104] used TBA3[TiO18Mo5(iPrO)] as precursor to obtain a series of new complexes; [TiO18Mo5(iOPr)]3-, [TiO18Mo5(iOBu)]3-, [TiO18Mo5(OMe)]3-, [TiO18Mo5(tBuO)]3-, [TiO18Mo5(4-MeC6H4O)]3-, [TiO18Mo5(2-CHOC6H4)]3-, and the dimer [(µ-O)(TiO18Mo5)2]6-, varying only in the set of ligands. No applications were reported.
An interesting example of a Mo-TOA was reported by Eslava et al. [60]. They solvothermally reacted [Ti(OEt)4]4 with MoCl5 in ethanol and obtained [(Ti4O8Mo2(OEt)10)2]. It features a central Mo4O10 framework with the relatively short distance (2.609 Å) between the molybdenum ions that suggests Mo-Mo bonding within the complex. The Mo-core was encapsulated by a layer of titanium atoms, connected via oxo and bridging ethoxide ligands. This complex had an extended absorption of visible light in the 400 nm to 550 nm. Several Mo-TOAs were synthesized by Uchiyama et al. [25] by reacting [Ti(iOPr)4] with MoO(Me)4 or MoO(iOPr)4 in toluene, which afforded the complexes [Ti2O4Mo2(OMe)6(iOPr)6], [Ti6O22Mo6(iOPr)16(iPrOH)2], and [Mo7O31+xTi7+x(OiPr)8+2x]. Annealing of the Mo7Ti7 compound at 500 °C afforded TiMoO5 as major phase, with minor impurities of anatase-TiO2, Ti6O11, and MoO3. It showed promising result for use as anode material in Li ion batteries. A selection of Mo-TOAs is shown in Fig. 21.
4.5 Cadmium
A variety of Cd-TOAs have been reported, from trinuclear Ti2Cd up to the spherical Ti17Cd2. An early Cd-TOA was produced by Veith and co-workers [105] as part of their series of [MTi2(iOPr)9] structures. Reacting [KTi2(iOPr)9] with CdI2 in toluene yielded [Ti2{CdI}(iOPr)9], though no application was reported. Wu et al. [106] prepared the doubly doped cadmium-containing complex [Ti17O28{CdIIPhen}2(iOPr)18] of peripheral type from reaction between [Ti(iOPr)4] and Cd(OAc)2 dihydrate in isopropanol. Electrodes were prepared by coating of ITO glass with dissolved complex and the photocurrent response was investigated. It displayed very poor photocurrent response in comparison to the [Ti17O28{CoIIPhen}2(iOPr)18] analogue, explained by poor redox properties of Cd. Artner and co-workers [92] reported a methacrylate-substituted Cd-TOA from reaction of equimolar amounts of [Ti(iOPr)4] and Cd(CH3COO)2 with 10 eq. of methacrylic acid in CH2Cl2. This afforded [Ti2O2Cd4(CH3COO)2(OMc)10(iPrOH)2]. The cadmium octahedra are connected via corner and edge-sharing. The titanium ions are connected via bridging methacrylate ligands and one bridging µ3-oxygen, connecting one Ti4+ to two different Cd2+ ions. A similar Cd-TOA, but with inverted core-structure, was prepared by Liu and colleagues [107]. Solvothermal reaction between [Ti(iOPr)4] and 0.7 eq. Cd(NO3)2 hexahydrate in acetonitrile with excess tert-butylacetic acid (tBA) gave H2[Ti4O4Cd2(tBA)12(iOPr)2]. Here, the Cd2+ ions are connected to the Ti4O2-core via bridging tBA ligands and µ3-oxygens, connecting one Cd2+ with two different titanium ions. The complex did not absorb in the visible region according to solid-state UV-vis measurements. The band gap was determined to 3.39 eV. The authors investigated hydrogen production under a 300 W xenon lamp by dispersing powder of H2[Ti4O4Cd2(tBA)12(iOPr)2] in water. A hydrogen production of 3.15 μmol h−1 was reported. Operational stability was claimed by comparing PXRD patterns before and after reaction. However, the reported PXRD patterns displayed substantial differences. A recent work reported a series of structures based on the (Ti10Cd2O8) core functionalized with a series of salicylate derivatives [108] from reaction between [Ti(iOPr)4] with Cd(NO3)2.4H2O (Ti:Cd ratio 6.5) in ethanol and heated at 60 °C for a week. All complexes had improved visible absorption up to ca. 600 nm, probably due to the salicylate ligands. Band gaps were reported to vary between 2.07 and 2.32 eV. Photocurrent responses of the Cd-TOAs were mostly improved, up to ca. 3 µA cm−2 compared to the undoped reference complex [Ti12O(4-MeSal)8(OEt)16], ca. 0.8 µA cm−2. Photoelectrodes were prepared by suspending the complexes in a water: ethanol solution containing Nafion followed by ultrasonication, and subsequent drop-casting on ITO electrodes. Complex stabilities were evaluated by recording PXRD patterns before and after soaking crystals in water for 24 h. While some of the complexes retained fairly similar diffraction patterns, others showed distinct changes. A selection of Cd-TOAs is shown in Fig. 22.
Structures of selected Cd-TOAs. a [Ti2{CdI}(iOPr)9]. b [Ti17O28{CoIIPhen}2(iOPr)18]. c [Ti2O2Cd4(CH3COO)2(OMc)10(iPrOH)2]. d H2[Ti4O4Cd2(tBA)12(iOPr)2]. Hydrogen atoms and alkoxide ligands have been removed for clarity. Cream is cadmium, green is titanium, red is oxygen, purple is iodine, light blue is nitrogen, and grey is carbon
5 Summary
To date, an extensive library of heterometallic titanium oxo-alkoxide complexes has been reported. In some cases, isostructural series have been synthesized simply by changing the dopant precursor. These compounds are very interesting for studying decomposition into oxide materials. Based on the doped TOAs reported so far, a few trends may be discerned. First, with a few exceptions (e.g. the polyoxometalate-like Mo-TOAs), most reported TOAs containing two or more transition metal dopants are stabilized by bridging or chelating heteroligands such as carboxylates or β-diketonates. While singly doped TOAs generally can exists as only (oxo)-alkoxy complexes, the dopant transition metal commonly retains one halide ion if a transition metal halide was used as precursor. Secondly, the reported TOA structures modified with second-row transition metals tend to have higher numbers of dopants compared to the first-row transition metals. However, the identity of the modifying ligands may have an influence on this and will require further investigation.
While the doped TOAs generally are considered attractive single-source precursors for complex oxides and doped titania, much fewer studies have actually focused on obtaining the materials. A few successful examples include ZrTi2O6, NiTiO3, CoTiO3, ZnTiO3, and manganese-doped rutile. As synthetic details are available for a large number of potential single source precursors, some focus should be directed toward systematic investigations (e.g., temperature, ligand, solvent effects) on their conversion into oxide materials.
References
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37. https://doi.org/10.1038/238037a0
Cargnello M, Gordon TR, Murray CB (2014) Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem Rev 114(19):9319–9345. https://doi.org/10.1021/cr500170p
Zhang RY, Elzatahry AA, Al-Deyab SS, Zhao DY (2012) Mesoporous titania: From synthesis to application. Nano Today 7(4):344–366. https://doi.org/10.1016/j.nantod.2012.06.012
Nakata K, Fujishima A (2012) TiO2 photocatalysis: Design and applications. J Photoch Photobio C 13(3):169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem Rev 107(7):2891–2959. https://doi.org/10.1021/cr0500535
Paz Y (2010) Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl Catal B-Environ 99(3–4):448–460. https://doi.org/10.1016/j.apcatb.2010.05.011
Teoh WY, Scott JA, Amal R (2012) Progress in heterogeneous photocatalysis: From classical radical chemistry to engineering nanomaterials and solar reactors. J Phys Chem Lett 3(5):629–639. https://doi.org/10.1021/jz3000646
Ochiai T, Fujishima A (2012) Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J Photoch Photobio C 13(4):247–262. https://doi.org/10.1016/j.jphotochemrev.2012.07.001
Oregan B, Gratzel M (1991) A Low-Cost, High-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740. https://doi.org/10.1038/353737a0
Fagan R, McCormack DE, Dionysiou DD, Pillai SC (2016) A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mat Sci Semicon Proc 42:2–14. https://doi.org/10.1016/j.mssp.2015.07.052
Maity P, Mohammed OF, Katsiev K, Idriss H (2018) Study of the bulk charge carrier dynamics in anatase and rutile TiO2 single crystals by femtosecond time-resolved spectroscopy. J Phys Chem C 122(16):8925–8932. https://doi.org/10.1021/acs.jpcc.8b00256
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B-Environ 125:331–349. https://doi.org/10.1016/j.apcatb.2012.05.036
Gribb AA, Banfield JF (1997) Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Am Miner 82(7-8):717–728. https://doi.org/10.2138/am-1997-7-809
Zhang HZ, Banfield JF (2005) Size dependence of the kinetic rate constant for phase transformation in TiO2 nanoparticles. Chem Mater 17(13):3421–3425. https://doi.org/10.1021/cm0508423
Weber AS, Grady AM, Koodali RT (2012) Lanthanide modified semiconductor photocatalysts. Catal Sci Technol 2(4):683–693. https://doi.org/10.1039/c2cy00552b
Okazaki M, Wang YA, Yokoi T, Maeda K (2019) Visible-light-driven water oxidation using anatase titania modified with first-row transition-metal-oxide nanoclusters. J Phys Chem C 123(16):10429–10434. https://doi.org/10.1021/acs.jpcc.9b01222
Zhang SB, Zhao YC, Yang JP, Zhang Y, Sun P, Yu XH, Zhang JY, Zheng CG (2017) Simultaneous NO and mercury removal over MnOx/TiO2 catalyst in different atmospheres. Fuel Process Technol 166:282–290. https://doi.org/10.1016/j.fuproc.2017.06.011
Hinojosa-Reyes M, Camposeco-Solis R, Ruiz F, Rodriguez-Gonzalez V, Moctezuma E (2019) Promotional effect of metal doping on nanostructured TiO2 during the photocatalytic degradation of 4-chlorophenol and naproxen sodium as pollutants. Mat Sci Semicon Proc 100:130–139. https://doi.org/10.1016/j.mssp.2019.04.050
Toloman D, Popa A, Stefan M, Pana O, Silipas TD, Macavei S, Barbu-Tudoran L (2017) Impact of Gd ions from the lattice of TiO2 nanoparticles on the formation of reactive oxygen species during the degradation of RhB under visible light irradiation. Mat Sci Semicon Proc 71:61–68. https://doi.org/10.1016/j.mssp.2017.07.004
Smirnova N, Petrik I, Vorobets V, Kolbasov G, Eremenko A (2017) Sol-gel synthesis, photo- and electrocatalytic properties of mesoporous TiO2 modified with transition metal ions. Nanoscale Res Lett 12:239. https://doi.org/10.1186/s11671-017-2002-3
Rozman N, Tobaldi DM, Cvelbar U, Puliyalil H, Labrincha JA, Legat A, Sever Skapin A (2019) Hydrothermal synthesis of rare-earth modified titania: Influence on phase composition, optical properties, and photocatalytic activity. Materials 12:713. https://doi.org/10.3390/ma12050713
Svensson FG, Cojocaru B, Qiu Z, Parvulescu V, Edvinsson T, Seisenbaeva GA, Tiseanu C, Kessler VG (2021) Rare-earth-modified titania nanoparticles: molecular insight into synthesis and photochemical properties. Inorg Chem 60(19):14820–14830. https://doi.org/10.1021/acs.inorgchem.1c02134
Shalan AE, Rashad MM (2013) Incorporation of Mn2+ and Co2+ to TiO2 nanoparticles and the performance of dye-sensitized solar cells. Appl Surf Sci 283:975–981. https://doi.org/10.1016/j.apsusc.2013.07.055
Ehsan MA, Naeem R, Khaledi H, Sohail M, Saeed AH, Mazhar M (2016) Fabrication of CoTiO3-TiO2 composite films from a heterobimetallic single source precursor for electrochemical sensing of dopamine. Dalton Trans 45(25):10222–10232. https://doi.org/10.1039/c6dt01016d
Uchiyama H, Puthusseri D, Grins J, Gribble D, Seisenbaeva GA, Pol VG, Kessler VG (2021) Single-source alkoxide precursor approach to titanium molybdate, TiMoO5, and its structure, electrochemical properties, and potential as an anode material for alkali metal ion batteries. Inorg Chem 60(6):3593–3603. https://doi.org/10.1021/acs.inorgchem.0c03087
Spijksma GI, Huiskes C, Benes NE, Kruidhof H, Blank DHA, Kessler VG, Bouwmeester HJM (2006) Microporous zirconia-titania composite membranes derived from diethanolamine-modified precursors. Adv Mater 18(16):2165-+. https://doi.org/10.1002/adma.200502568
Lu HJ, Wright DS, Pike SD (2020) The use of mixed-metal single source precursors for the synthesis of complex metal oxides. Chem Commun 56(6):854–871. https://doi.org/10.1039/c9cc06258k
Mishra S, Jeanneau E, Tian L, Nuta I, Blanquet E, Singh D, Ahuja R, Marichy C, Daniele S (2022) Asymmetry-induced redistribution in Sn(IV)-Ti(IV) hetero-bimetallic alkoxide precursors and its impact on thin-film deposition by metal-organic chemical vapor deposition. Cryst Growth Des 22(1):54–59. https://doi.org/10.1021/acs.cgd.1c01136
Matthews PD, King TC, Wright DS (2014) Structure, photochemistry and applications of metal-doped polyoxotitanium alkoxide cages. Chem Commun 50(85):12815–12823. https://doi.org/10.1039/c4cc04421e
Cotton FA (1964) Metal atom clusters in oxide systems. Inorg Chem 3:1217–1220
Wright DA, Williams DA (1968) The crystal and molecular structure of titanium tetramethoxide Acta Crystallographica Section B B24:1107–1114
Ibers JA (1963) Crystal and molecular structure of titanium(IV) ethoxide. Nature 197:686–687
Bradley D, Methora RC, Rothwell I, Singh A (2001) Alkoxo and aryloxo derivatives of metals. Academic Press, San Diego
Kessler VG, Spijksma GI, Seisenbaeva GA, Hakansson S, Blank DHA, Bouwmeester HJM (2006) New insight in the role of modifying ligands in the sol-gel processing of metal alkoxide precursors: A possibility to approach new classes of materials. J Sol-Gel Sci Techn 40(2-3):163–179. https://doi.org/10.1007/s10971-006-9209-6
Rozes L, Sanchez C (2011) Titanium oxo-clusters: precursors for a Lego-like construction of nanostructured hybrid materials. Chem Soc Rev 40(2):1006–1030. https://doi.org/10.1039/c0cs00137f
Blanchard J, Ribot F, Sanchez C, Bellot PV, Trokiner A (2000) Structural characterization of titanium-oxo-polymers synthesized in the presence of protons or complexing ligands as inhibitors. J Non-Cryst Solids 265(1-2):83–97. https://doi.org/10.1016/S0022-3093(99)00885-6
L.G. H-P (1992) Metal alkoxides and β-diketonates as precursors for oxide and non-oxide thin films. Appl Organomet Chem 6:627–643
Rozes L, Steunou N, Fornasieri G, Sanchez C (2006) Titanium-oxo clusters, versatile nanobuilding blocks for the design of advanced hybrid materials. Monatsh Chem 137(5):501–528. https://doi.org/10.1007/s00706-006-0464-6
Leaustic A, Babonneau F, Livage J (1989) structural investigation of the hydrolysis-condensation process of titanium alkoxides Ti(OR)(4) (OR = OPri 2, OEt) modified by acetylacetone. 2. from the modified precursor to the colloids. Chem Mater 1(2):248–252. https://doi.org/10.1021/cm00002a016
Scolan E, Sanchez C (1998) Synthesis and characterization of surface-protected nanocrystalline titania particles. Chem Mater 10(10):3217–3223. https://doi.org/10.1021/cm980322q
Doeuff S, Henry M, Sanchez C, Livage J (1987) Hydrolysis of titanium alkoxides - modification of the molecular precursor by acetic-acid. J Non-Cryst Solids 89(1-2):206–216. https://doi.org/10.1016/S0022-3093(87)80333-2
Mutin PH, Guerrero G, Vioux A (2005) Hybrid materials from organo coupling molecules. J Mater Chem 100:205–223
Hayami R, Gunji T (2021) A review of phosphorous(V)-substituted titanium oxo-clusters. J Sol-Gel Sci Techn 100:205–223
Schubert U (2021) Titanium-oxo clusters with bi- and tridentate organic ligands: gradual evolution of the structures from small to big. Chem-Eur J 27(44):11239–11256. https://doi.org/10.1002/chem.202101287
Seisenbaeva GA, Moloney MP, Tekoriute R, Hardy-Dessources A, Nedelec JM, Gun’ko YK, Kessler VG (2010) Biomimetic synthesis of hierarchically porous nanostructured metal oxide microparticles-potential scaffolds for drug delivery and catalysis. Langmuir 26(12):9809–9817. https://doi.org/10.1021/la1000683
Svensson FG, Daniel G, Tai CW, Seisenbaeva GA, Kessler VG (2020) Titanium phosphonate oxo-alkoxide “clusters”: solution stability and facile hydrolytic transformation into nano titania. RSC Adv 10(12):6873–6883. https://doi.org/10.1039/c9ra10691j
Svensson FG, Seisenbaeva GA, Kessler VG (2017) Mixed-ligand titanium “oxo clusters”: structural insights into the formation and binding of organic molecules and transformation into oxide nanostructures on hydrolysis and thermolysis. Eur J Inorg Chem 35:4117–4122. https://doi.org/10.1002/ejic.201700775
Kessler VG (2009) The chemistry behind the sol-gel synthesis of complex oxide nanoparticles for bio-imaging applications. J Sol-Gel Sci Techn 51(3):264–271. https://doi.org/10.1007/s10971-009-1946-x
Mendez MS, Jia ZX, Traore M, Ben Amar M, Nikravech M, Kanaev A (2021) Nucleation and growth of mixed vanadium-titanium oxo-alkoxy nanoparticles in sol-gel synthesis. Colloid Surf A 610:125636. https://doi.org/10.1016/j.colsurfa.2020.125636
Cheng K, Chhor K, Kanaev A (2017) Solvent effect on nucleation-growth of titanium-oxo-alkoxy nanoparticles. Chem Phys Lett 672:119–123. https://doi.org/10.1016/j.cplett.2017.01.059
Van Lokeren L, Maheut G, Ribot F, Escax V, Verbruggen I, Sanchez C, Martins JC, Biesemans M, Willem R (2007) Characterization of titanium dioxide nanoparticles dispersed in organic ligand solutions by using a diffusion-ordered spectroscopy-based strategy. Chem-Eur J 13(24):6957–6966. https://doi.org/10.1002/chem.200601722
Barjat H, Morris GA, Smart S, Swanson AG, Williams SCR (1995) High-resolution diffusion-ordered 2D spectroscopy (HR-DOSY) - a new tool for the analysis of complex-mixtures. J Magn Reson Ser B 108(2):170–172. https://doi.org/10.1006/jmrb.1995.1118
Svensson FG, Kessler VG (2020) Solid-state structure and solution behavior of two titanium oxo-alkoxide complexes with phenylphosphonate ligands. Polyhedron 178:114276. https://doi.org/10.1016/j.poly.2019.114276
Seisenbaeva GA, Daniel G, Nedelec JM, Gun’ko YK, Kessler VG (2012) High surface area ordered mesoporous nano-titania by a rapid surfactant-free approach. J Mater Chem 22(38):20374–20380. https://doi.org/10.1039/c2jm33977c
Turova NY, Turevskaya EP, Kessler VG, Yanovskaya MI (1994) Oxoalkoxides-true precursors of complex oxides. J Sol-Gel Sci Techn 2(1-3):17–23. https://doi.org/10.1007/Bf00486207
Turova NY (2004) Metal oxoalkoxides. synthesis, properties and structures. Usp Khim+ 73(11):1131–1154
Doeuff S, Henry M, Sanchez C, Livage J (1987) The gel route to Cr3+-doped TiO2 an ESR study. J Non-Cryst Solids 89:84–97
VanGelder LE, Brennessel WW, Matson EM (2018) Tuning the redox profiles of polyoxovanadate-alkoxide clusters via heterometal installation: toward designer redox Reagents. Dalton Trans 47(11):3698–3704. https://doi.org/10.1039/c7dt04455k
VanGelder LE, Forrestel PL, Brennessel WW, Matson EM (2018) Site-selectivity in the halogenation of titanium-functionalized polyoxovanadate-alkoxide clusters. Chem Commun 54(50):6839–6842. https://doi.org/10.1039/c8cc01517a
Eslava S, Goodwill BPR, McPartlin M, Wright DS (2011) Extending the family of titanium heterometallic-oxo-alkoxy cages. Inorg Chem 50(12):5655–5662. https://doi.org/10.1021/ic200350j
Yi XY, Zhang QF, Lam TCH, Chan EYY, Williams ID, Leung WH (2006) Phosphato, chromato, and perrhenato complexes of titanium(IV) and zirconium(IV) containing Klaui’s tripodal ligand. Inorg Chem 45(1):328–335. https://doi.org/10.1021/ic051329u
Zhang JX, Hu W, Zhang J, Liu SJ, Tong J, Hou XD, Liu WL, Yang JL, Liu B (2017) Stable heteropolyoxotitanate nanocluster for full solar spectrum photocatalytic hydrogen evolution. J Phys Chem C 121(34):18326–18332. https://doi.org/10.1021/acs.jpcc.7b04064
Jerzykiewicz LB, Utko J, John L, Duczmal M, Sobota P (2007) Titanium-manganese compound with a chiral Mn3Ti center. Inorg Chem 46(22):9024–9026. https://doi.org/10.1021/ic7013094
Eslava S, McPartlin M, Thomson RI, Rawson JM, Wright DS (2010) Single-source materials for metal-doped titanium oxide: syntheses, structures, and properties of a series of heterometallic transition-metal titanium oxo cages. Inorg Chem 49(24):11532–11540. https://doi.org/10.1021/ic101687m
Li N, Matthews PD, Leung JJ, King TC, Wood PT, Luo HK, Wright DS (2015) Synthesis, structure and properties of the manganese-doped polyoxotitanate cage [Ti18MnO30(OEt)(20)(MnPhen)(3)](Phen=1,10-phenanthroline). Dalton Trans 44(44):19090–19096. https://doi.org/10.1039/c5dt03617h
Chen Y, Jarzembska KN, Trzop E, Zhang L, Coppens P (2015) How does substitutional doping affect visible light absorption in a series of homodisperse Ti-11 polyoxotitanate nanoparticles? Chem-Eur J 21(32):11538–11544. https://doi.org/10.1002/chem.201500961
Yang S, Su HC, Hou JL, Luo W, Zou DH, Zhu QY, Dai J (2017) The effects of transition-metal doping and chromophore anchoring on the photocurrent response of titanium-oxo-clusters. Dalton Trans 46(29):9639–9645. https://doi.org/10.1039/c7dt01603d
Chen Y, Trzop E, Makal A, Chen YS, Coppens P (2013) A novel manganese-doped large polyoxotitanate nanocluster. Dalton Trans 43:3839–3841
Lv YK, Cheng J, Matthews PD, Holgado JP, Willkomm J, Leskes M, Steiner A, Fenske D, King TC, Wood PT, Gan LH, Lambert RM, Wright DS (2014) A study of the optical properties of metal-doped polyoxotitanium cages and the relationship to metal-doped titania. Dalton Trans 43(23):8679–8689. https://doi.org/10.1039/c4dt00555d
Chen Y, Sokolow JD, Trzop E, Coppens P (2013) A manganese-doped polymeric framework of polyoxotitanate nanoclusters with a narrow band gap. Dalton Trans 42(43):15285–15287. https://doi.org/10.1039/c3dt52218k
Zhang K, Lin P, Du SW (2020) Facile and scalable synthesis of Ti6Mn2 oxo-cluster nanocrystals with flower-like morphology and excellent photocatalytic properties. Dalton Trans 49(8):2444–2451. https://doi.org/10.1039/c9dt04886c
Yu JG, Xiang QJ, Zhou MH (2009) Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl Catal B-Environ 90(3–4):595–602. https://doi.org/10.1016/j.apcatb.2009.04.021
Andriamiadamanana C, Laberty-Robert C, Sougrati MT, Casale S, Davoisne C, Patra S, Sauvage F (2014) Room-temperature synthesis of iron-doped anatase TiO2 for lithium-ion batteries and photocatalysis. Inorg Chem 53(19):10129–10139. https://doi.org/10.1021/ic501067p
Nunes GG, Reis DM, Amorim PT, Sa EL, Mangrich AS, Evans DJ, Hitchcock PB, Leigh GJ, Nunes FS, Soares JF (2002) Novel double alkoxides of titanium(IV) and iron(I)/(III): synthetic, structural and spectroscopic studies. N. J Chem 26(5):519–522. https://doi.org/10.1039/b111517k
Camargo PHC, Nunes GG, de Sa EL, Tremiliosi-Filho G, Evans DJ, Zarbin AJG, Soares JF (2008) Synthesis of Fe/Ti oxides from a single source alkoxide precursor under inert atmosphere. J Braz Chem Soc 19(8):1501–1512. https://doi.org/10.1590/S0103-50532008000800009
Jarzembska KN, Chen Y, Nasca JN, Trzop E, Watson DF, Coppens P (2014) Relating structure and photoelectrochemical properties: electron injection by structurally and theoretically characterized transition metal-doped phenanthroline-polyoxotitanate nanoparticles. Phys Chem Chem Phys 16(30):15792–15795. https://doi.org/10.1039/c4cp02509a
Eslava S, Hengesbach F, McPartlin M, Wright DS (2010) Heterometallic cobalt(II)-titanium(IV) oxo cages; key building blocks for hybrid materials. Chem Commun 46(26):4701–4703. https://doi.org/10.1039/c0cc00016g
Lai YH, Lin CY, Lv YK, King TC, Steiner A, Muresan NM, Gan LH, Wright DS, Reisner E (2013) Facile assembly of an efficient CoOx water oxidation electrocatalyst from Co-containing polyoxotitanate nanocages. Chem Commun 49(39):4331–4333. https://doi.org/10.1039/c2cc34934e
Lv Y, Willkomm J, Steiner A, Gan LH, Reisner E, Wright DS (2012) Encapsulation of a ‘naked’ Br- anion in a polyoxotitanate host. Chem Sci 3(8):2470–2473. https://doi.org/10.1039/c2sc20193c
Wang C, Liu C, Li LJ, Sun ZM (2019) Synthesis, crystal structures, and photochemical properties of a family of heterometallic titanium oxo clusters. Inorg Chem 58(9):6312–6319. https://doi.org/10.1021/acs.inorgchem.9b00508
Lv YK, Cheng J, Steiner A, Gan LH, Wright DS (2014) Dipole-induced band-gap reduction in an inorganic cage. Angew Chem Int Ed 53(7):1934–1938. https://doi.org/10.1002/anie.201307721
Seisenbaeva GA, Mallah T, Kessler VG (2010) Highly symmetric organic ligand-capped Lindqvist structures derived from 3d-elements. Dalton Trans 39(33):7774–7779. https://doi.org/10.1039/c0dt00275e
Tahir AA, Mazhar M, Hamid M, Wijayantha KGU, Molloy KC (2009) Photooxidation of water by NiTiO3 deposited from single source precursor [Ni2Ti2(OEt)(2)(mu-OEt)(6)(acac)(4)] by AACVD Dalton Trans 19:3674–3680. https://doi.org/10.1039/b818765g
Hu JY, Zhan LJ, Zhang GY, Zhang Q, Du L, Tung CH, Wang YF (2016) Effects of substitutional dopants on the photoresponse of a polyoxotitanate cluster. Inorg Chem 55(17):8493–8501. https://doi.org/10.1021/acs.inorgchem.6b01071
Wang C, Lu YJ, Rao MY, Chen N, Wang SJ, Kong FG (2021) Co-crystal of Ti4Ni2 and Ti8Ni4 clusters with enhanced photochemical properties. CrystEngComm 23(24):4402–4407. https://doi.org/10.1039/d1ce00369k
Veith M, Mathur S, Huch V (1997) Synthesis and characterization of new alkoxotitanates of yttrium, barium, and copper: Single crystal x-ray diffraction structures of Cl2Y{Ti-2(OPri)(9)}, {Ti(OPri)(5)}Ba{Ti-2(OPri)(9)}, and ClCu{Ti-2(OPri)(9)}. Inorg Chem 36(11):2391–2399. https://doi.org/10.1021/ic9613218
Hamid M, Tahir AA, Mazhar M, Zeller M, Hunter AD (2007) Heterobimetallic molecular cages for the deposition of Cu/Ti and Cu/Zn mixed-metal oxides. Inorg Chem 46(10):4120–4127. https://doi.org/10.1021/ic0624470
Liu CD, Wu YX, Meng XY, Zhao A, Shao YL, Liu W, Huang X, Pan FX, Liu WS (2020) Construction of two novel titanium oxide clusters: copper ion introducing enhances photocatalytic performance. Eur J Inorg Chem 2020(44):4189–4194. https://doi.org/10.1002/ejic.202000715
Artner C, Koyun A, Schubert U (2015) A heterobimetallic copper-titanium oxo cluster with a new structural motif. Monatsh Chem 146(11):1777–1780. https://doi.org/10.1007/s00706-015-1558-9
Petrus R, Chomiak K, Utko J, Wilk-Kozubek M, Lis T, Cybinska J, Sobota P (2020) Convenient route to heterometallic group 4-zinc precursors for binary oxide nanomaterials. Inorg Chem 59(12):8108–8120. https://doi.org/10.1021/acs.inorgchem.0c00399
Petrus R, Drag-Jarzabek A, Utko J, Bykowski D, Lis T, Sobota P (2017) Molecular routes to group iv magnesium and calcium nanocrystalline ceramics. Inorg Chem 56(18):11365–11374. https://doi.org/10.1021/acs.inorgchem.7b01772
Artner C, Koyun A, Czakler M, Schubert U (2014) Mixed-metal oxo clusters structurally derived from Ti6O4(OR)(8)(OOCR’)(8). Eur J Inorg Chem 29:5008–5014. https://doi.org/10.1002/ejic.201402499
Garden JA, White AJP, Williams CK (2017) Heterodinuclear titanium/zinc catalysis: Synthesis, characterization and activity for CO2/epoxide copolymerization and cyclic ester polymerization. Dalton Trans 46(8):2532–2541. https://doi.org/10.1039/c6dt04193k
Ehsan MA, Khaledi H, Pandikumar A, Rameshkumar P, Huang NM, Arifin Z, Mazhar M (2015) Nitrite ion sensing properties of ZnTiO3-TiO2 composite thin films deposited from a zinc-titanium molecular complex. N. J Chem 39(9):7442–7452. https://doi.org/10.1039/c5nj00850f
Jupa M, Kickelbick G, Schubert U (2004) Methacrylate-substituted titanium-yttrium mixed-metal oxo clusters. Eur J Inorg Chem 9:1835–1839. https://doi.org/10.1002/ejic.200300694
Zechmann CA, Huffman JC, Folting K, Caulton KG (1998) Controlled stoichiometric incorporation of Ti(IV) into a Zr(IV) pinacolate. Inorg Chem 37(22):5856–5861. https://doi.org/10.1021/ic980714z
Gainsford GJ, Al-Salim N, Kemmitt T (2002) A novel titanium-mu-O-zirconium complex: bis(mu-methyliminodiethanolato-1 kappa O-3,N,O ‘;1: 2 kappa O-2)(methyliminodiethanolato-2 kappa O-3,N,O ‘)dipropanolato-1 kappa O,2 kappa O-titanium(IV)- zirconium(IV). Acta Crystallogr C 58:M509–M510. https://doi.org/10.1107/S0108270102015275
Gainsford GJ, Al-Salim N, Kemmitt T (2002) A centrosymmetric triply alkoxo-bridged titanium-mu(2)-O-zirconium tetranuclearcomplex: [TiZr(mu(2)-O,mu(2)-O ‘-methyliminodiethanolate)(mu(2)-O-n-propanolate)2-(n-propanolate)(2.7)(isopropanolate)(1.3)](2). ActaCrystallogr E58:M636–M638 https://doi.org/10.1107/S1600536802018482
Moraru B, Kickelbick G, Schubert U (2001) Methacrylate-substituted mixed-metal clusters derived from zigzag chains of [ZrO8]/[ZrO7] and [TiO6] polyhedra. Eur J Inorg Chem 5:1295–1301
Kickelbick G, Feth MP, Bertagnolli H, Moraru B, Trimmel G, Schubert U (2002) EXAFS investigations on nanocomposites composed of surface-modified zirconium and zirconium/titanium mixed metal oxo clusters and organic polymers. Monatsh Chem 133(6):919–929. https://doi.org/10.1007/s007060200062
Spijksma GI, Bouwmeester HJM, Blank DHA, Kessler VG (2004) Molecular design approach to a stable heterometallic zirconium-titanium alkoxide - potential precursor of mixed-oxide ceramics. Inorg Chem Commun 7(8):953–955. https://doi.org/10.1016/j.inoche.2004.06.006
Yuan LB, He YP, Zhang L, Zhang J (2018) Embonic acid functionalized niobium complexes with selective dye sorption properties. Inorg Chem 57(8):4226–4229. https://doi.org/10.1021/acs.inorgchem.7b03265
Errington RJ, Coyle L, Middleton PS, Murphy CJ, Clegg W, Harrington RW (2010) Synthesis and Structure of the Alkoxido-TitaniumPentamolybdate ((Bu4N)-Bu-II)(3)[((PrO)-Pr-i)TiMo5O18]: An Entry into Systematic TiMo5 Reactivity J Clust Sci 21(3):503–514. https://doi.org/10.1007/s10876-010-0329-3
Coyle L, Middleton PS, Murphy CJ, Clegg W, Harrington RW, Errington RJ (2012) Protonolysis of [((PrO)-Pr-i)TiMo5O18](3-):Access to a family of TiMo5 Lindqvist type polyoxometalates Dalton Trans 41(3):971–981. https://doi.org/10.1039/c1dt11256b
Veith M, Mathur S, Huch V (1996) Synthesis and Spectroscopic Characterization of Novel Heterotermetallic Isopropoxides: X-ray Crystal Structures of ICd{M2(OPri)9} and [{Cd(OPri)3}Ba{M2(OPri)9}]2 (M) Ti, Hf). Inorg Chem 35:7295–7303. https://doi.org/10.1021/ic960116p
Wu YY, Wang P, Wang YH, Jiang JB, Bian GQ, Zhu QY, Dai J (2013) Metal-phenanthroline fused Ti-17 clusters, a single molecular source for sensitized photoconductive films. J Mater Chem A 1(34):9862–9868. https://doi.org/10.1039/c3ta11571b
Liu JX, Zeng XC, Zhang L, Zhang J (2016) A new cadmium-doped titanium-oxo cluster with stable photocatalytic H-2 evolution properties. Dalton Trans 45(11):4501–4503. https://doi.org/10.1039/c6dt00333h
Wu YX, Liu XR, Chen G, Tian YQ, Yan J, Yi XY, Liu C (2021) Cd-doped polyoxotitanium nanoclusters with a modifiable organic shell for photoelectrochemical water splitting. Inorg Chem 60(24):19263–19269. https://doi.org/10.1021/acs.inorgchem.1c03078
Svensson FG (2020) Hybrid nano titania: Molecular formation mechanisms and applications in nanotechnology. Doctoral Thesis. https://pub.epsilon.slu.se/17878/
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svensson, F.G. Structural diversity in transition metal-doped titanium oxo-alkoxy complexes: Potential sol-gel intermediates for doped titania nanoparticles and complex titanates. J Sol-Gel Sci Technol 103, 595–615 (2022). https://doi.org/10.1007/s10971-022-05847-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05847-4