Abstract
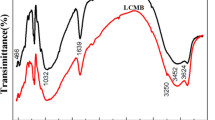
The generation of nuclear energy produces a large amount of uranium containing wastewater, which must be treated. In this study, amino functionalized bentonite (Bent-NH2) was prepared by modifing Gaomiaozi bentonite with 3-Aminopropyltriethoxysilane. Various characterization results confirmed the successful grafting. The batch adsorption experiments indicated Bent-NH2 reached its maximum adsorption capacity rapidly (20 min), 49.168 mg/g. Notably, Bent-NH2 had better adsorption performance than unmodified bentonite in really groundwater with different pH. This work demonstrates that Bent-NH2 has excellent adsorption performance and can be used for low uranium wastewater treatment and geological disposal.













Similar content being viewed by others
References
Liu H, Fu T, Sarwar MT, Yang H (2023) Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl Clay Sci 232:106796. https://doi.org/10.1016/j.clay.2022.106796
Sun Y, Yang S, Sheng G, Guo Z, Wang X (2012) The removal of U(VI) from aqueous solution by oxidized multiwalled carbon nanotubes. J Environ Radioact 105:40–47. https://doi.org/10.1016/j.jenvrad.2011.10.009
Hu W, Lu S, Song W, Chen T, Hayat T, Alsaedi NS (2018) Competitive adsorption of U(VI) and Co(II) on montmorillonite: a batch and spectroscopic approach - sciencedirect. Appl Clay Sci 157:121–129. https://doi.org/10.1016/j.clay.2018.02.030
Arnquist IJ, Vacri Mhoppe EW (2020) An automated ultra clean ion exchange separation method for the determinations of 232Th and 238U in copper using inductively coupled plasma mass spectrometry. Nucl Instrum Meth B 965:163761. https://doi.org/10.1016/j.nima.2020.163761
Maheswari M, Asubramanian MS (2004) Selective enrichment of U(VI), Th(IV) and La(III) from high acidic streams using a new chelating ion-exchange polymeric matrix. Talanta 64(1):202–209. https://doi.org/10.1016/j.talanta.2004.02.029
Chen F, Lv M, Ye Y, Miao S, Tang X, Liu Y (2022) Insights on uranium removal by ion exchange columns: the deactivation mechanisms, and an overlooked biological pathway. Chem Eng J 434:134708. https://doi.org/10.1016/j.cej.2022.134708
Karmakar R, Sen K (2019) Role of biomolecules in selective extraction of U(VI) using an aqueous biphasic system. J Radioanal Nucl Ch 322:57–66. https://doi.org/10.1007/s10967-019-06494-w
Fu M, Ao J, Ma L, Kong D, Qi S, Zhang P (2022) Uranium removal from waste water of the tailings with functional recycled plastic membrane. Sep Purif Technol 287:120572. https://doi.org/10.1016/j.seppur.2022.120572
Saloua J, Mohamed T, Ahmed M, Khadija M (2020) Industrial rejection: removal of heavy metals based on chemical precipitation and research for recoverable material in byproducts. Int J Manag Sci Eng. 7(2):39–52
Özbek YY, Erdem Baştan F, Canikoğlu N (2016) The experimental study of titanium-ions into hydroxyapatite by chemical precipitation. J Therm Anal Calorim 125:651–658. https://doi.org/10.1007/s10973-016-5335-8
Cheira MF, Kouraim MN, Zidan IH, Mohamed WS, Hassanein TF (2020) Adsorption of U(VI) from sulfate solution using montmorillonite/polyamide and nano-titanium oxide/polyamide nanocomposites. J Environ Chem Eng 8(5):104427. https://doi.org/10.1016/j.jece.2020.104427
Wang Y, Cui W, Wang H, Xie Y, Liu Y (2020) Adsorption of U(VI) on sulfonated ordered mesoporous polymer. J Radioanal Nucl Ch 1:1–12. https://doi.org/10.1007/s10967-020-07322-2
Chikkamath S, Patel MA, Kar AS, Tomar BS, Manjanna J (2023) Diffusion of radioisotopes in fe-montmorillonite relevant to geological disposal of hlw. Phys Chem Earth 131:103429. https://doi.org/10.1016/j.pce.2023.103429
Ibrahim HA, El-Kamash AM, Hanafy Mabdel-Monem NM (2008) Examination of the use of synthetic zeolite naa-x blend as backfill material in a radioactive waste disposal facility: thermodynamic approach. Chem Eng J 144(1):67–74. https://doi.org/10.1016/j.cej.2008.01.012
Zong P, Wu X, Gou J, Lei X, Liu D, Deng H (2015) Immobilization and recovery of uranium(vi) using na-bentonite from aqueous medium: equilibrium, kinetics and thermodynamics studies. J Mol Liq 209:358–366. https://doi.org/10.1016/j.molliq.2015.05.052
Qi LY, Yang XY, Wang CL (2018) Sorption Behavior of 125I- on Gaomiaozi Bentonite. J Radioanal Nucl Ch 40(2):112–120. https://doi.org/10.7538/hhx.2017.yx.2017016
Jia W, Lu S (2014) Effect of ph, foreign ions and temperature on radionickel sorption onto bentonite from inner mongolia, china. J Radioanal Nucl Ch 299(3):1417–1426. https://doi.org/10.1007/s10967-013-2865-4
Lu S, Xu H, Wang M, Song X, Liu Q (2012) Sorption of Eu(III) onto gaomiaozi bentonite by batch technique as a function of pH, ionic strength, and humic acid. J Radioanal Nucl Ch 292(2):889–895. https://doi.org/10.1007/s10967-011-1532-x
Chen YG, Ye WM, Yang XM, He DY (2011) Effect of contact time, ph, and ionic strength on Cd(II) adsorption from aqueous solution onto bentonite from gaomiaozi, china. Environ Earth Sci 64(2):329–336. https://doi.org/10.1007/s12665-010-0850-6
Sun Z, Chen Y, Mu X, Wu D, Ye W (2021) Graphene oxide-modified organic gaomiaozi bentonite for Yb(III) adsorption from aqueous solutions. Mater Chem Phys 274:125176. https://doi.org/10.1016/j.matchemphys.2021.125176
Shi S, Liu J, Shu J, Wu P, Zhao C, Liu N, Lan T (2023) Uranium(VI) adsorption from carbonate solutions using cetyltrimethylammonium bromide modified purified-bentonite-mof composite. Appl Clay Sci 241:106986. https://doi.org/10.1016/j.clay.2023.106986
Liu J, Shi S, Li C, Hong X, Gu Z, Li F, Liu C (2021) U(VI) adsorption by one-step hydrothermally synthesized cetyltrimethylammonium bromide modified hydroxyapatite-bentonite composites from phosphate-carbonate coexisted solution. Appl Clay Sci 203:106027. https://doi.org/10.1016/j.clay.2021.106027
Anirudhan TS, Lekshmi GS, Shainy F (2019) Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J Colloid Interface Sci 534:248–261. https://doi.org/10.1016/j.jcis.2018.09.009
You J, Wang L, Zhao Y, Bao W (2021) A review of amino-functionalized magnetic nanoparticles for water treatment: features and prospects. J Clean Prod 281:124668. https://doi.org/10.1016/j.jclepro.2020.124668
Meng B, Guo Q, Men X, Ren S, Jin W, Shen B (2020) Modified bentonite by polyhedral oligomeric silsesquioxane and quaternary ammonium salt and adsorption characteristics for dye. J Saudi Chem Soc 24(3):334–344. https://doi.org/10.1016/j.jscs.2020.01.007
Tobilko V, Spasonova L, Kovalchuk I, Kornilovych B (2019) Adsorption of uranium(VI) from aqueous solutions by amino-functionalized clay minerals. Colloid Interfac 3(1):41. https://doi.org/10.3390/colloids3010041
Li S, Wang X, Huang Z, Du L, Tan Z, Fu Y, Wang X (2016) Sorption and desorption of uranium(VI) on GMS bentonite: effect of ph, ionic strength, foreign ions and humic substances. J Radioanal Nucl CH 308(3):877–886. https://doi.org/10.1007/s10967-015-4513-7
Xiao J, Chen Y, Zhao W, Xu J (2013) Sorption behavior of U(VI) onto chinese bentonite: effect of ph, ionic strength, temperature and humic acid. J Mol Liq 188:178–185. https://doi.org/10.1016/j.molliq.2013.10.008
Kostenko LS, Tomashchuk II, Kovalchuk TV, Zaporozhets OA (2019) Bentonites with grafted aminogroups: synthesis, protolytic properties and assessing Cu(II), Cd(II) and pb(II) adsorption capacity. Appl Clay Sci 172(MAY):49–56. https://doi.org/10.1016/j.clay.2019.02.009
Zhen Y, Li ZD, Pan YM (2019) Enhanced adsorption of cationic pb(II) and anionic Cr(VI) ions in aqueous solution by amino-modified nano-sized illite-smectite clay. Environ Sci Pollut R 26(11):11126–11139. https://doi.org/10.1007/s11356-019-04447-0
Cheng J, Leng Y, Gu R, Yang G, Tuo X (2021) Adsorption of uranium(VI) from groundwater by amino-functionalized clay. J Radioanal Nucl Chem 327:1365–1373. https://doi.org/10.1007/s10967-021-07617-y
Piscitelli F, Posocco P, Toth R, Fermeglia M, Pricl S, Mensitieri G, Lavorgna M (2010) Sodium montmorillonite silylation: unexpected effect of the aminosilane chain length. J Colloid Interf Sci 351(1):108–115. https://doi.org/10.1016/j.jcis.2010.07.059
Mckay Y (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Zhao D, Yang S, Chen S, Guo Z, Yang X (2020) Correction to: effect of ph, ionic strength and humic substances on the adsorption of uranium(VI) onto na-rectorite. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06994-9
Wang X, Fan Q, Yu S, Chen Z, Wang X (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455. https://doi.org/10.1016/j.cej.2015.11.066
Ren X, Wang S, Li Y (2010) Influence of contact time, ph, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto mx-80 bentonite. J Radioanal Nucl Ch 283(1):253–259. https://doi.org/10.1007/s10967-009-0323-0
Boussouga Y, Joseph J, Stryhanyuk H, Richnow H (2023) Adsorption of uranium (vi) complexes with polymer-based spherical activated carbon. Water Res. https://doi.org/10.1016/j.watres.2023.120825
Priya S, Ilaiyaraja P, Priyadarshini N, Subalekha N (2023) Effective removal of uranium(VI) using magnetic acid functionalized Fe3O4@TiO2 nanocomposite from aqueous solutions. Process Saf Environ Protect 178:480–493. https://doi.org/10.1016/j.psep.2023.08.034
Ilaiyaraja P, Deb A, Sponraju D (2015) Removal of uranium and thorium from aqueous solution by ultrafiltration (UF) and pamam dendrimer assisted ultrafiltration (DAUF). J Radioanal Nucl Chem 303(1):441–450. https://doi.org/10.1007/s10967-014-3462-x
Zhou L, Ouyang J, Liu Z, Huang G, Wang Y, Li Z, Adesina A (2019) Highly efficient sorption of U(VI) from aqueous solution using amino/amine-functionalized magnetic mesoporous silica nanospheres. J Radioanal Nucl Chem 319:987–995. https://doi.org/10.1007/s10967-018-6381-4
Li Q, Yue QY, Sun HJ, Su Y, Gao B (2010) A comparative study on the properties, mechanisms and process designs for the adsorption of non-ionic or anionic dyes onto cationic-polymer/bentonite. J Environ Manage 91(7):1601–1611. https://doi.org/10.1016/j.jenvman.2010.03.001
Liu J, Zhao C, Wang J, He H, Yuan G, Wang H (2019) Adsorption of U(VI) from eutrophic aquesous solutions in a U(VI)–P–CO3 system with hydrous titanium dioxide supported by polyacrylonitrile fiber. Hydrometallurgy 183:29–37. https://doi.org/10.1016/j.hydromet.2018.11.009
Parkhurst D L , Appelo C A J (1999) User's guide to phreeqc version 3—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-resources investigations report. 99(4259): 312. https://www.usgs.gov/software/phreeqc-version-3/
Li T, Xia L, Wang F (2022) Selective capture uranium from acidic solution by potassium manganese ferrocyanide. J Environ Chem Eng 10(6):108884. https://doi.org/10.1016/j.jece.2022.108884
Wang S, Zhao L, Yang H, Du S, Yang C, Lu B, Wang G (2023) Rational design phosphorylation mg-fe layered dioxides nanosheets for efficient capture of uranium from wastewater. Sep Purif Technol 326:124862. https://doi.org/10.1016/j.seppur.2023.124862
Langmuir I (2015) The adsorption of gases on plane surfaces of glass, mica and platinum. J Chem Phys 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Yu Y, Jiang D, He B, Yu B, Pu X, Liu D, Xiong W, Liu N, Yuan G (2023) Facile preparation of uio-66 derivatives for the removal of Co(II) from aqueous solution: study on adsorption properties and irradiation stability. J Radioanal Nucl Chem 332(10):4047–4056. https://doi.org/10.1007/s10967-023-09114-w
Limin Z, Jinbo O, Hamza S, Zhang GL (2018) Adsorption of U(VI) onto the carboxymethylated chitosan/na-bentonite membranes: kinetic, isothermic and thermodynamic studies. J Radioanal Nucl Chem 317:1–9. https://doi.org/10.1007/s10967-018-6009-8
Song W, Liu M, Hu R, Tan X, Li J (2014) Water-soluble polyacrylamide coated-Fe3O4 magnetic composites for high-efficient enrichment of U(VI) from radioactive wastewater. Chem Eng J 246:268–276. https://doi.org/10.1016/j.cej.2014.02.101
Guimar Es V, Rodríguez-Castellón E, Algarra M, Rocha Fbobos I (2016) Kinetics of uranyl ions sorption on heterogeneous smectite structure at ph4 and 6 using a continuous stirred flow-through reactor. Appl Clay Ence 134:71–82. https://doi.org/10.1016/j.clay.2016.03.028
Guimar Es V, Rodríguez-Castellón E, Algarra M, Rocha Fbobos I (2016) Influence of pH, layer charge location and crystal thickness distribution on U(VI) sorption onto heterogeneous dioctahedral smectite. J Hazard Mater 317:246–258. https://doi.org/10.1016/j.jhazmat.2016.05.060
Vodolazov LI, Shatalov VV, Molchanova TV, Peganov VA (2001) Polymerization of uranyl ions and its role in ion-exchange extraction of uranium. Atom Energy 90(3):213–217. https://doi.org/10.1023/a:1011372311373
Zhang J, Yan Z, Ouyang J, Yang H, Chen D (2018) Highly dispersed sepiolite-based organic modified nanofibers for enhanced adsorption of congo red. Appl Clay Ence 157:76–85. https://doi.org/10.1016/j.clay.2018.02.031
Duan SX, Wang YN, Liu X, Shao DD, Tawwar H (2017) Removal of U(VI) from aqueous solution by amino functionalized flake graphite prepared by plasma treatment. Acs Sustain Chem Eng 5(5):4073–4085. https://doi.org/10.1021/acssuschemeng.7b00069
Acknowledgements
This work has been supported by CAEA Innovation Center for Geological Disposal of High-Level Radioactive Waste Foundation (No. CXJJ2110-4) and Sichuan Science and Technology Program (No. 2023NSFSC1454) funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists in the submission of this manuscript, and the manuscript has been approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, J., Gu, R., Jiang, Q. et al. Highly efficient removal of uranium (VI) from aqueous solutions by amino functionalized bentonite. J Radioanal Nucl Chem 333, 1301–1314 (2024). https://doi.org/10.1007/s10967-023-09345-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09345-x