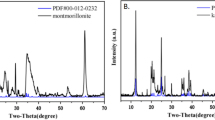
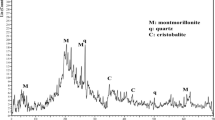
Abstract
A kind of amino-functionalized clay was prepared by grafting APTES from clay in the Alxa area (Inner Mongolia, China), and its application for the U(VI) removal from Alxa groundwater was studied in this work. The results indicate that the sorption strongly depended on the pH and ions. The presence of amino functional groups significantly enhances the adsorption of uranium on clay in groundwater. The sorption isotherms fitted well with the Langmuir model, whereas the sorption kinetics could be fitted by a pseudo-second-order model. These findings indicate that amino-functionalized clay can effectively adsorb U(VI) in complex groundwater samples.










Similar content being viewed by others
References
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X (2015) Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ Sci Technol 49(7):4255–4262
Wang XX, Fan QF, Yu SJ, Chen ZS, Ai YJ, Sun YB, Wang XK (2016) Retracted: high sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455
Sun YB, Li JX, Wang XK (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Xiao J, Chen Y, Zhao W, Xu J (2013) Sorption behavior of U(VI) onto Chinese bentonite: effect of pH, ionic strength, temperature and humic acid. J Mol Liq 188:178–185
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41(20):6182–6188
Ren XM, Wang SW, Yang ST, Li JX (2013) Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J Radioanal Nuclear Chem 283(1):253–259
Zarrougui R, Mdimagh R, Raouafi N (2017) Highly efficient extraction and selective separation of uranium(VI) from transition metals using new class of undiluted ionic liquids based on h-phosphonate anions. J Hazard Mater 342:464
Wang J, Chen Z, Shao D, Li Y, Hu S (2017) Adsorption of U(VI) on bentonite in simulation environmental conditions. J Mol Liq 242:678–684
Ye Z, Yin X, Chen L, He X, Wei Y (2019) An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J Clean Prod 236:117631
Kahraman HT, Pehlivan E (2019) Evaluation of anion-exchange resins on the removal of Cr(VI) polluted water: batch ion-exchange modeling. Arabian J Geosci 12(16):1–10
Kumar BB, Jadhav UU, Munusamy M, Lianghui J, En-Hua Y, Bin C (2018) Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries. ACS Sustain Chem Eng 6(9):12343–12352
Turanov AN, Karandashev VK, Baulin VE, Baulin DV (2019) Extraction of Re(III), U(VI), and Th(IV) from perchlorate solutions with tetraphenyl(o-oxyphenylenemethylene)diphosphine dioxide. Radiochemistry 61(2):156–161
Li P, Wang J, Wang Y, Liang J, He B, Pan D (2019) Photoconversion of u(VI) by TiO2: an efficient strategy for seawater uranium extraction. Chem Eng J 365(1):231–241
Jakovetić S, Luković N, Jugović B, Gvozdenović M, Grbavčić S, Jovanović J, Knežević-Jugović Z (2015) Production of antioxidant egg white hydrolysates in a continuous stirred tank enzyme reactor coupled with membrane separation unit. Food Bioprocess Technol 8(2):287–300
Zhao D, Yang S, Chen S, Guo Z, Yang X (2020) Correction to: effect of pH, ionic strength and humic substances on the adsorption of uranium(VI) onto Na-rectorite. J Radioanal Nuclear Chem 323(2):1013
Xu Z, Xing YX, Ren AR, Ma DD, Li YX, Hu SH (2020) Study on adsorption properties of water hyacinth-derived biochar for uranium(VI). J Radioanal Nucl Chem 324(3):1317–1327
Fan FL, Qin Z, Bai J, Rong WD, Fan FY, Tian W (2012) Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J Environ Radioact 106:40–46
Liu Y, Yuan L, Yuan Y, Lan J, Li Z, Feng Y (2012) A high efficient sorption of U(VI) from aqueous solution using amino-functionalized SBA-15. J Radioanal Nuclear Chem 292(2):803–810
Kausar A, Iqbal M, Javed A, Aftab K, Nazli ZIH, Bhatti HN (2018) Dyes adsorption using clay and modified clay: a review. J Mol Liq 256:395–407
Wang XL, Han ZH, Zhang LQ, Zhou J (2018) Regional engineering geology suitability assessment for high-level radioactive waste disposal of pre-selected Alxa area. J Eng Geol 26(6):1715–1723
Cheng JF, Leng YC, Lai J, Zhu S, Tuo XG (2017) Speciation and adsorption behavior of Pu in groundwater. Environ Chem 36(7):1630–1635
Al-Anber MA, Al-Momani IF, Zaitoun MA, Al-Qaisi W (2020) Inorganic silica gel functionalized tris(2-aminoethyl)amine moiety for capturing aqueous uranium(VI) ion. J Radioanal Nuclear Chem 325(1):605–623
Huynh J, Rubén P, Allavena A, Hervé G, Batonneau-Gener I (2020) Selective adsorption of U(VI) from real mine water using an NH2-functionalized silica packed column. Chem Eng J 405(1):126912
Tobilko V, Spasonova L, Kovalchuk I (2019) Adsorption of uranium(VI) from aqueous solutions by amino-functionalized clay minerals. Coll Interfaces 3(1):41
Li ZY, Pan ZD, Wang YM (2019) Enhanced adsorption of cationic Pb(II) and anionic Cr(VI) ions in aqueous solution by amino-modified nano-sized illite-smectite clay. Environ Sci Pollut Res Int 26(11):11126–11139
Asgari M, Abouelmagd A, Sundararaj U (2017) Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl Clay Sci 146:439–448
Moreira MA, Ciuffi KJ, Rives V, Vicente MA, Trujillano R, Gil A (2016) Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl Clay Sci 135(JAN.):394–404
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D (2010) Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci 349(1):293–299
Zhou LM, Ouyang JB, Shehzad H, Le ZG, Adesina AA (2018) Adsorption of U(VI) onto the carboxymethylated chitosan/Na-bentonite membranes: kinetic, isothermic and thermodynamic studies. J Radioanal Nucl Chem 317:1–9
Liu W, Zhang L, Chen F, Wang H, Wang Q, Liang K (2020) Efficiency and mechanism of adsorption of low-concentration uranium from water by a new chitosan/aluminum sludge composite aerogel. Dalton Trans 49(10):3209–3221
Zhang J, Zhai S, Li S, Xiao Z, Song Y, An Q (2013) Pb(II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel proce. Chem Eng J 215:461–471
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Zhou LM, Ouyang J, Liu Z, Huang G, Wang Y, Li Z (2019) Highly efficient sorption of U(VI) from aqueous solution using amino/amine-functionalized magnetic mesoporous silica nanospheres. J Radioanal Nucl Chem 319:987–995
Tan L, Tan X, Ren X, Mei H, Wang X (2018) Influence of pH, soil humic acid, ionic strength and temperature on sorption of U(VI) onto attapulgite. J Radioanal Nucl Chem 316:981–991
Li S, Wang X, Huang Z, Du L, Tan Z, Fu Y (2016) Sorption and desorption of uranium(VI) on GMZ bentonite: effect of pH, ionic strength, foreign ions and humic substances. J Radioanal Nuclear Chem 308(3):877–886
Atia AA (2005) Studies on the interaction of mercury(II) and uranyl(II) with modified chitosan resins. Hydrometallurgy 80(1–2):13–22
Shen H, Pan S, Zhang Y, Huang X, Gong H (2012) A new insight on the adsorption mechanism of amino-functionalized nano-Fe3O4 magnetic polymers in Cu(II), Cr(VI) co-existing water system. Chem Eng J 183:180–191
Bulut Y, Aydın H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194(1–3):259–267
Langmuir I (2015) The adsorption of gases on plane surfaces of glass, mica and platinum. J Chem Phys 40(9):1361–1403
Freundlich H (1907) Over the adsorption in solution. J Phys Chem 57(1):385–470
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochim Cosmochim Acta 70(6):1379–1387
Acknowledgements
Financial supports from National Natural Science Foundation of China Younth Fun (No. 41603124) and National Natural Science Foundation of China (No. 41630646) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, J., Leng, Y., Gu, R. et al. Adsorption of uranium(VI) from groundwater by amino-functionalized clay. J Radioanal Nucl Chem 327, 1365–1373 (2021). https://doi.org/10.1007/s10967-021-07617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07617-y