Abstract
This work assesses potential physicochemical, metallic, and radiological contamination of liquid discharges from the phosphoric acid (PA) production unit at the coast of El Jadida Province in Morocco. The physicochemical parameters: pH, conductivity, salinity, turbidity, total hardness, nitrate, nitrite, orthophosphate, and heavy metals were analyzed in PA and beach samples. 238U, 232Th, and 226Ra were determined by gamma spectrometry, the Radon contents were determined using solid state detectors (LR-115). It is concluded that phosphate effluents are strongly acidic with a mean pH-value of 1.8 and that the discharges still contain relatively high levels of fluoride, phosphorus and radiological substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water pollution is among the most challenging issues in today’s world. El Jadida Province in Morocco is characterized by urban agglomerations and important industrial infrastructure such as the phosphoric acid production unit at the coast that processes phosphate rock using sulfuric acid as shown in Eq. (1) [1].
The production of phosphoric acid generates relatively large volumes of phosphogypsum. Approximately 20 million metric tonnes phosphogypsum are produced at the phosphoric acid production unit in El Jadida every year [2]. The untreated phosphogypsum is directly discharged into coastal waters in form of a liquid slurry. In addition to this effluent, various gases are produced during the digestion of phosphate rock with sulfuric acid that are also emitted into the environment.
The disposal of phosphogypsum into the sea is still conducted in Lebanon [3], Tunisia [4, 5, 6, 7] and Morocco. Worldwide approximately 58% of the produced phosphogypsum is stacked, 28% is discharged in coastal waters, and only 14% is further processed [8]. OCP, the state-owned Moroccan phosphate rock mining company and phosphoric acid manufacturer, wants to stop the practice of seawater phosphogypsum disposal in the near future [9] and is actively working on methods to valorize phosphogypsum instead of disposing it [10]. The material could indeed be source of valuable secondary raw materials such as gypsum [11] and rare earth elements [12,13,14], with the monetary costs for mining already covered by the the phosphate industry [15]. As of now the environmental effects of discharging phosphogypsum into Moroccan coastal waters are not fully understood yet.
Azouazi et al. [16] were probably the first to provide a systematic analysis of the radioactivity found in Moroccan Khouribga phosphate rock, resulting phosphogypsum and potential transfer of the radioactive elements into groundwaters from dry stacking the phosphogypsum on land (when this practice was still used in Morocco). More recent analysis of Khouribga phosphate rock that is processed in El-Jadida Province as well as the resulting phosphogypsum are available [17,18,19] and since the practice of dry-stacking phosphogypsum was not continued several studies focused their attention on analyzing coastal samples.
Erramli et al. [20] analyzed six solid samples (including one sample taken at the beach of Safi City south of El-Jadida) and eight water samples from the sewers of the fertilizer plant (not the discharged phosphogypsum). The study largely focused on comparing different analyzing techniques without commenting on potential pollution of the analyzed samples. Belahbib et al. [21] conducted the most recent and most systematic analysis drawing sand and seawater samples from 17 locations north and south of the discharge point. The study focused on the release of radioactive material with the phosphogypsum slurry and found a considerable increase of the 226Ra activity in beach sand that ranged from 16 Bq/kg to as much as 217 Bq/kg. The authors could further show that the contamination is present in a diameter as large as 10 km from the discharge point.
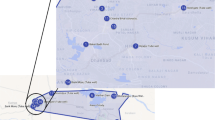
The increase in radioactivity at beaches surrounding the discharge point is obviously of great concern. There is a larger number of other parameters that are of interest as well and the present study builds on the earlier work from Belahbib et al. [21] but analysis seawater directly and considers additional parameters. The pH, turbidity, conductivity, TDS (total dissolved solids), TOC (total organic carbon), heavy metals, and radioactive elements were determined for six samples collected at two sample locations north-west of the discharge point (S1: beach near Moulay Abdellah) and south-east of the discharge point (S2: beach near Sidi Abed) as indicated in Fig. 2. Besides, this work provides a comprehensive analysis of the phosphate rock that was processed, the resulting phosphogypsum and the resulting phosphoric acid before and after concentration. In addition, the liquid waste streams from the phosphoric acid production unit could for the first time be directly analyzed. Specifically, the study analyzed the phosphogypsum slurry as the principal effluent (PE) from the phosphoric acid production unit, the effluent from the cooling channels of the phosphoric acid plant (CC) and the effluent of the phosphoric acid concentration unit (CPA). All samples were collected in February 2021.
Materials and methods
Samples from the phosphoric acid production unit
Figure 1 provides a simplified schematic overview of the phosphoric acid production unit in El Jadida with the three main effluents that have been analyzed as part of this study. The phosphoric acid production plant uses seawater for cooling in a thermal power plant that provides steam to the sulfuric acid plant. The sulfuric acid is subsequently used in the phosphoric acid plant. All three parts of the facility (thermal power plant, sulfuric acid plant, and phosphoric acid plant) use seawater as a coolant and that coolant (CC in Fig. 1) was subsequently analyzed in this work.
The principal effluent analyzed in the work (PE in Fig. 1) is the phosphogypsum slurry that is directly discharged into the sea. After phosphate rock is processed with sulfuric acid to phosphoric acid as shown in Eq. 1 the phosphogypsum crystals are separated from the phosphoric acid using tilting cell filters as indicated in Fig. 1. The remainder is discharged into a waste pit from where the phosphogypsum slurry is ultimately disposed of into the sea. Strong ocean currents are then supposed to disperse the phosphogypsum.
The phosphoric acid produced in the phosphoric acid workshop has a P2O5 concentration of approximately 29% and still needs to be further concentrated to 54%. This step is done in the concentration unit where the acid is first heated in the acid heater to reach the boiling point of water at the operating pressure to generate steam in the boiler. During water evaporation in the boiler, the vapors formed (or mist) mainly contain water, but also minor traces of acid and other impurities such as fluorinated compounds. The vapors circulate towards the condenser. The condensable gases that are contained in the steam condense in contact with a seawater film that is used for cooling. The seawater, containing the condensed steam, is then evacuated to a hydraulic guard as indicated in Fig. 1. The overflow from the hydraulic guard is again directed to the tailing pit and another effluent that is ultimately discharged into the sea. We labeled this effluent CPA and also analyzed it in this work.
Samples near Moulay Abdellah and Sidi Abed
Besides taking samples from the phosphoric acid unit directly six seawater samples were taken at two sampling locations. Three samples were taken near Moulay Abdellah (S1) that is located 16 km northeast of the discharge point and three samples were taken near Sidi Abed (S2) that is located 11 km southwest of the discharge point (see Fig. 2). The average value of each measurement of the three samples is presented. In addition, two sand samples and two mussel (Mytilus galloprovincialis) samples were taken near Moulay Abdellah (S1) and Sidi Abed (S2). Sand and seawater samples were taken from the wave breaking zone and mussels were collected at low tide, in the intertidal zone in the lower mediolittoral zone, and then transported to the laboratory where they were brushed, cleaned, and stripped of their epibionts before they were sorted by size class in 5 mm intervals.
Measurement of physicochemical parameters
Following early experience of Melki and Gueddari [22] the collected samples were acidified with 0.1 N HNO3 and stored at 4 °C before they were analyzed. Physicochemical parameters such as the pH, electrical conductivity (EC), turbidity, salinity, alkalinity (TAC), oxidizability, total hardness (TH), chloride (Cl−), sulphate, ammonia (NH4), nitrite (NO2), and nitrate (NO3) were determined. In addition, major elements such as orthophosphates (P2O5), fluorine (F), and trace metals were determined.
The pH, EC, and TAC were directly determined at the sample location with portable instruments. A turbidity meter similar to the work described by Rahmanian et al. [23] was used to determine the turbidity. Individual samples were poured into sample holders in which they were kept (usually for a few minutes) until reading stability was achieved. After achieving reading stability, the value was recorded. Total hardness (TH), chloride content, and sulphate content were determined using the Mohr volumetric method [24]. Orthophosphates, ammonia, nitrite, and nitrate were analyzed using UV–Visible spectrophotometry, and the fluoride ion concentrations were measured with an ion selective electrode. Trace elements were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) with external calibration and internal standard correction.
Activity measurements
The phosphate rock, phosphoric acid (pre-concentrated and concentrated), phosphogypsum, effluents and seawater, sand and mussel samples were analyzed using gamma spectrometry as described in an earlier work by Arhouni et al. [10]. The analyses were carried out using a broad energy germanium (BEGe) detector for the determination of 238U, 226Ra, 232Th and 235U activity concentration. The concentrations of 214Pb (295 keV and 352 keV) and 214Bi (609 keV, 1120 keV, and 1764 keV) were used to quantify the activity of 226Ra assuming that the secular equilibrium is established between 226Ra and 222Rn during the measurement (226Ra can be challending to distinguish from 235U because interference from emitting a photon at energy levels close to 186.1 keV and 185.72 keV). 228Ac (911 keV and 969 keV peaks) and 212Pb (239 keV peak) concentrations were used to quantify the 232Th activity.
A resolution of 1.8 keV with an energy measurement of 1332.5 keV was achieved. A certified multi-energy standard (same conditions and geometry as the samples) was used for calibration of a broad energy Ge detector. This standard contained a liquid multi-gamma source containing several radioelements (large energy range) whose activities are certified by the supplier. Besides, three reference materials provided by the International Atomic Energy Agency (IAEA) were chosen for calibration purposes.
Experimental set-up to measure radon and the exhalation rate
The radon concentration and exhalation rates were determined by passive alpha dosimetry with 2 × 2 cm2 pieces of LR115 type 2 detector material from Kodak (Rochester, USA) as previously described in detail by Boukhair et al. [25] and Taoufiq et al. [26]. The samples were conditioned in radon-tight containers to control radon emanation [27] for at least 4 weeks to establish a secular equilibrium that corresponds to seven half-lives of 222Rn.
Results and discussion
Major and trace element analysis
Data obtained for the major element and trace element concentrations in the phosphate rock (PR), phosphogypsum (PG), and phosphoric acid (PA) before concentration (PA-BC) and after concentration (PA-AC) are given in Table 1. The Khouribga phosphate rock used here shows a relatively high P2O5 content of 30.59%, a relatively low Al2O3 content of 0.43%, and a relatively low total silica content of 2.25%. Trace elements in the phosphate rock are the main source of impurities that further transfer to the phosphoric acid. During the manufacturing of phosphoric acid, the impurities originally contained in the phosphate rock are distributed between the phosphoric acid and the phosphogypsum. The results in Table 1 show that overall more than 2/3 of the impurities pass into the phosphoric acid. The major element contents in the phosphoric acid are phosphorus, aluminum, iron, and magnesium. In addition, other trace elements such as cadmium, chrome, copper, vanadium, zinc and uranium largely transfer to the phosphoric acid.
The phosphogypsum is usually sent as a pulp into the seawater. The solubility of the phosphogypsum in seawater is pH dependent, and it is according to Guo et al. [28] generally highly soluble in saltwater reaching a value of 4.1 g/L. There are two types of impurities in the phosphogypsum that can be differentiated. Soluble impurities, composed of salts or acids not eliminated by washing in the process, such as P2O5 and fluorine as well as particles that are responsible for the low pH of phosphogypsum (pH < 3). The other group of impurities are insoluble impurities that come from the phosphate ore and do not react with the phosphoric acid. Silica, major trace elements and heavy metals are examples of the second group of impurities.
Acidity as a result of hydrofluorosilicic acid (hexafluorosilicic acid) is one of the issues raised by Fertilizers Europe [29]. This acid reacts with seawater salt as following:
The salt dissolves in the water, whereas the HCl acidifies the medium. Due to the large volume of seawater and the intense marine flow on the coast of Morocco, this acidity cannot be easily detected, even when only being tens of meters away from the discharge point. The same conclusion is reached when it comes to radioactive elements and lime sulphate. Insoluble products are present in very low concentrations and are widely dispersed by the seawater at the discharge point.
Table 2 summarizes the variations of the pH, P2O5, and heavy metals concentrations in the samples collected from the liquid discharges of the phosphoric acid production unit (PE, CC and CPA).
The pH of the cooling channel sample was found to be slightly acidic with a value of 6.2. However, the pH in the samples collected from the outlet of the concentration unit and the PG was only 1.8 showing that both substances are highly acidic. According to the United States Environmental Protection Agency [30], the acceptable pH that is suitable for the protection of aquatic habitats ranges between 6.5–9.0.
In the wet phosphoric acid industry, the main objective is to extract the maximum of P2O5. Losses of phosphorus may occur after processing when phosphorus is consumed and excreted, or during processing by the impregnation of co-crystallized P2O5 in the crystal lattice of gypsum or throughout the condensation of the vapor obtained as a result of the concentration of the phosphoric acid. These losses affect the overall performance of the phosphoric acid production units. Analysis of phosphorus compounds have shown that these effluents present higher phosphorus concentrations. It was further eminent that the effluents were polluted with fluoride (concentrations varied from 3.28 to 927 mg/L). Fluoride has both beneficial and detrimental effects on human health. At low doses, fluoride prevents dental caries, at high doses (> 1.5 mg/L) [31] it causes problems such as dental and bone fluorosis.
Heavy metals are a major pollutant found in phosphogypsum [32]. The highest concentrations of the majority of metals (Fe, Zn, and Cr) were recorded at the phosphogypsum discharge (PE). The obtained results are, however, within the permissible limit set by the Moroccan Standards [33] for direct discharges of heavy metals into aquatic environments (surface waters). The maximum concentrations set by these standards are: 0.5 mg/L for Pb, 2 mg/L for Cr, 0.5 mg/L for Cu and 0.2 mg/L for Cd [33].
Physicochemical parameters
To assess the effect of the industrial effluents on the marine environment, the common physicochemical parameters of water pH, electrical conductivity, turbidity, salinity, and total hardness have been determined at two locations (S1 and S2). Results are listed in Table 3.
The results show that the seawater from all locations was moderately alkaline (pH 7.0–7.9) but within the permissible national limit of pH 6.5–9 [33]. Low pH levels can promote heavy metal solubility.
The measured conductivity provides insights into pollution with inorganic materials [34]. The conductivity in the seawater samples is in the order of 48.3 mS/cm. The measured values were within the allowable level of conductivity which is in the order of 50 mS/cm [33].
Turbidity refers to the content of suspended particles in water that disturb it. The values obtained of turbidity varied in the range of 0.92 to 5.27 NTU. The measured values were within the national allowable limit which is in the order of 5 NTU [33].
Salinity generally describes the total concentration of the dissolved inorganic ions in a medium with increases in salinity as a result of anthropogenic activity referred to as secondary salinization [35]. Since both values measured at S1 and S2 are the same we did not measure secondary salinization in this work.
In the present study the total hardness (sum of calcium and magnesium concentration) of the seawater samples was found to be 7.4 °F. The samples can thus be classified as soft following the classification of Shariati-Rad and Heidari [36].
The chloride content of marine water is similar in both locations. The results show higher chloride ion concentrations in the seawater samples. This augmentation could be caused by a non-negligible contribution of chemicals, specifically phosphogypsum discharged at these two sites.
Nitrites are generally less abundant than nitrates. They are formed as a result of organic matter degradation, nitrate reduction, and ammonia oxidation. Nitrite concentrations are less than 0.10 mg/L in this work and are below the Moroccan standard limit of 0.5 mg/L [33].
Table 4 shows the concentrations of heavy metals in the seawater samples collected near Moulay Abdellah (S1) and Sidi Abbed (S2). The highest values of P2O5 and fluorine were recorded at the Sidi Abed beach while the lowest are recorded for the Moulay Abdellah beach. All obtained concentrations are within the permissible limits.
Radioactivity measurements
Radiological characterization of the radionuclides contained in the phosphate rock (PR), phosphogypsum (PG), phosphoric acid (PA), before concentration (BC) as well as after concentration (AC) and seawater effluents have been performed to provide a database for the assessment of the environmental and the radiological impacts of the phosphoric acid unit. Table 5 shows the specific activities of the main radionuclides of the three radioactive families (238U, 232Th, and 235U) present in these samples. Most samples showed concentrations that were below the detection limit.
The sedimentary phosphate rock from Morocco treated for the production of phosphoric acid is clearly enriched in radionuclides from the uranium series, which is also well-known [37, 38]. Furthermore, in the phosphate rock, all radionuclides of the uranium series are in secular equilibrium (Fig. 3), as expected given that the material undergoes no radionuclide concentration during the physical processes used prior to digestion. Phosphate rock (PR) from Morocco contains among many other accompanying elements natural uranium in relatively high concentrations (1048 Bq/kg). During the processing of phosphate rock with sulfuric acid, the majority (892 Bq/kg) of the radiotoxic Ra present in phosphate rock transfers to the phosphogypsum replacing Ca in the chemical structure [39, 40]. As a result of the relatively low radioactivity of phosphogypsum the material is usually classified as the TENORM (Technically Enhanced Naturally Occurring Radioactive Material) residue and not a radioactive waste under most national regulations [41,42,43]. If phosphogypsum is stacked on land it is often considered to be a serious environmental liability though [43, 44]. Seawater disposal should not be considered as a serious alternative since the environmental risks associated with it are too little understood. This study and an earlier work by Jia et al. [45] that looked into the effects of roughly 30 years of phosphogypsum seawater disposal in Italy indicate that the practice does, however, disperse the phosphogypsum to a degree that most activity is well below the detection limit.
The variation in radon activity and exhalation rate of each effluent sample type is shown in Table 6. The highest value of the radon activity and exhalation rate was recorded for the phosphogypsum slurry (PE), with a value in the order of 509 Bq/m3.
The phosphogypsum discharge has a direct impact on approximately 150 km of coastline and beaches in the Moroccan province of El Jadida. This radioactive pollution has the potential to harm marine and coastal ecosystems, as well as the livelihoods of entire communities. Among aquatic organisms, mussels extract and concentrate elements from their surroundings but lack the ability to release radioisotopes from their bodies. In a study [21] conducted in 2019 in the two stations, Mytilus galloprovincialis mussels were analysed since they are filter feeders and thus effective bio-accumulators that can act as reliable indicator of radioactive elements in water over longer periods of time. In addition to mussels, the volume activities of radon in coastal sand samples was evaluated because this sand is widely used in the construction industry in the El Jadida region and other provinces. Results of beach sands and mussels samples from S1 and S2 that were evaluated as part of this work are presented in Table 7.
The average volume activities of radon in sand and mussel samples at station 1, which is located an average distance of 16 km north of the discharge point, are in the order of 707 and 286 Bq/m3, respectively. Whereas, in station 2, at a perimeter of 10 km, the average activities of radon in sand and mussel samples increased significantly to 6,066 and 897 Bq/m3. The radiological impact is significant, and it has the potential to harm humans and the environment. Even though Moulay Abdellah is indeed closer to the main discharge point, the radiological impact has remained low. This was most likely due to the discharge being dispersed by the dominant north–south sea current.
To better understand the results in Tables 6 and 7 the radon activity and exhalation rate of the phosphate rock (PR), phophogypsum (PG) as well as the phosphoric acid (PA) before concentration (BC) and after concentration (AC) were determined and are depicted in Fig. 4. The phosphogypsum showed a radon activity of approximately 2005 Bq/m3 that explains the relatively high activity value in the principal effluent. The seawater at the Sidi Abed beach shows higher radiation levels than the seawater drawn from the Moulay Abdellah beach which is not surprising given the different distances from the discharge point. Both values remain within the permissible limit (11,100 mBq/m3) set by the World Health Organization [31] and it can thus be assumed that the phosphate effluents do not present a relevant radiological risk during their release into the aquatic environment in Morocco.
Conclusions
The effluents from the phosphoric acid production unit in El Jadida Province in Morocco contain considerable amounts of heavy metals and radiological substances. The effluents are further highly acidic and can contain high levels of fluoride and phosphorus. Seawater samples taken at two locations (Moulay Abdellah and Sidi Abed) indicate that the concentrations are within permissible limits. We nonetheless urge the practice of direct seawater discharge to stop and to find better ways to valorize phosphogypsum and the other effluents rather than disposing them into the sea. Although within permissible limits we strongly believe that with phosphogypsum dilution should not be the solution to pollution.
References
Becker P (1989) Phosphates and phosphoric acid: raw materials, Technology and Economics of the Wet Processes, 2nd edn. Marcel Decker Inc., New York
Bouargane B, Laaboubi K, Biyoune MG et al (2023) Effective and innovative procedures to use phosphogypsum waste in different application domains: review of the environmental, economic challenges and life cycle assessment. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-023-01617-8
Aoun M, Arnaudguilhem C, El Samad O et al (2015) Impact of a phosphate fertilizer plant on the contamination of marine biota by heavy elements. Environ Sci Pollut Res 22:14940–14949. https://doi.org/10.1007/s11356-015-4691-4
Boudaya L, Mosbahi N, Dauvin J-C, Neifar L (2019) Structure of the benthic macrofauna of an anthropogenic influenced area: Skhira Bay (Gulf of Gabès, central Mediterranean Sea). Environ Sci Pollut Res 26:13522–13538. https://doi.org/10.1007/s11356-019-04809-8
El Zrelli R, Rabaoui L, Daghbouj N et al (2018) Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories (SE Tunisia): high mining potential and implications for environmental protection. Environ Sci Pollut Res 25:14690–14702. https://doi.org/10.1007/s11356-018-1648-4
Ben Salem Z, Ayadi H (2017) First investigation of trace metal distribution in surface seawater and copepods of the south coast of Sfax (Tunisia). Environ Sci Pollut Res 24:19662–19670. https://doi.org/10.1007/s11356-017-9536-x
El Zrelli R, Baliteau JY, Yacoubi L, Castet S, Gregoire M et al. (2021) Rare earth elements characterization associated to the phosphate fertilizer plants of Gabes (Tunisia Central Mediterranean Sea): Geochemical properties and behavior related economic losses and potential hazards. Science of The Total Environment 791, 148268. https://doi.org/10.1016/j.scitotenv.2021.148268
Bilal E, Bellefqih H, Bourgier V et al (2023) Phosphogypsum circular economy considerations: a critical review from more than 65 storage sites worldwide. J Clean Prod 414:137561. https://doi.org/10.1016/j.jclepro.2023.137561
Hermann L, Kraus F, Hermann R (2018) Phosphorus processing—potentials for higher efficiency. Sustainability 10(5):1482. https://doi.org/10.3390/su10051482
Arhouni FE, Hakkar M, Mahrou A et al (2022) Better filterability and reduced radioactivity of phosphogypsum during phosphoric acid production in Morocco using a fly ash waste and pure silica additive. J Radioanal Nucl Chem 331:1609–1617. https://doi.org/10.1007/s10967-022-08235-y
Haneklaus N, Barbossa S, Basallote MD et al (2022) Closing the upcoming EU gypsum gap with phosphogypsum. Resour Conserv Recycl 182:106328. https://doi.org/10.1016/j.resconrec.2022.106328
Ait Brahim J, Amal M, Mazouz H, Redouane B (2023) Recovery of rare earth elements and sulfuric acid solution from phosphate byproducts via hydrofluoric acid conversion. Journal of Industrial and Engineering Chemistry. https://doi.org/10.1016/j.jiec.2023.07.028
Diwa RR, Tabora EU, Haneklaus NH, Ramirez JD (2023) Rare earths leaching from Philippine phosphogypsum using Taguchi method regression and artificial neural network analysis. Journal of Material Cycles and Waste Management. https://doi.org/10.1007/s10163-023-01753-1
Al Khaledi N, Taha M, Hussein A, Hussein E, El Yahyaoui A, Haneklaus N (2019) Direct leaching of rare earth elements and uranium from phosphate rocks. IOP Conf. Ser.: Mater. Sci. Eng. 479 012065. https://doi.org/10.1088/1757-899X/479/1/012065
Reitsma F, Woods P, Fairclough M, Kim Y, Tulsidas H et al (2018) On the Sustainability and Progress of Energy Neutral Mineral Processing. Sustainability 10(1):235. https://doi.org/10.3390/su10010235
Azouazi M, Ouahidi Y, Fakhi S et al (2001) Natural radioactivity in phosphates, phosphogypsum and natural waters in Morocco. J Environ Radioact 54(2):231–242. https://doi.org/10.1016/S0265-931X(00)00153-3
Agayr K, Chanouri H, Achiou B et al (2022) Study on the kinetics of the conversion of Moroccan phosphogypsum into X2SO4 (X = Na, NH4). J Mater Cycles Waste Manag 24(5):2015–2029. https://doi.org/10.1007/s10163-022-01461-2
Hakkar M, Arhouni FE, Mahrou A et al (2021) Enhancing rare earth element transfer from phosphate rock to phosphoric acid using an inexpensive fly ash additive. Minerals Eng 172:107166. https://doi.org/10.1016/j.mineng.2021.107166
Qamouche K, Chetaine A, El Yahyaoui A et al (2021) Uranium and other heavy metal sorption from Moroccan phosphoric acid with argan nutshell sawdust. Minerals Eng 171:107085. https://doi.org/10.1016/j.mineng.2021.107085
Erramli H, Misdaq GHD, MA, et al (2008) Study of pollution in the El Jadida-Safi Atlantic coastal zone (Morocco) by using PIXE and SSNTD methods. J Environ Radioact 99(8):1216–1223. https://doi.org/10.1016/j.jenvrad.2008.02.003
Belahbib L, Arhouni FE, Boukhair A et al (2021) Impact of phosphate industry on natural radioactivity in sediment, seawater, and coastal marine fauna of El Jadida province, Morocco. J Hazard Toxic Radioact Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000563
Melki S, Gueddari M (2017) Impact assessment of phosphogypsum on the groundwater of sfax-agarebaquifer, in southeast of Tunisia. World Academy of Science, Engineering and Technology International Journal of Energy and Power Engineering 11(7)
Rahmanian N, Bt Ali SH, Homayoonfard M et al (2015) Analysis of physiochemical parameters to evaluate the drinking water quality in the state of Perak, Malaysia. J Chem 2015:716125. https://doi.org/10.1155/2015/716125
Rodier J, Legube B, Merlet I (2009) L’analyse de l’eau. 9ème édition, Dunod, Paris
Boukhair A, Belahbib L, Azkour K et al (2016) Measurement of natural radioactivity and radon 503 exhalation rate in coal ash samples from a thermal power plant. World J Nucl Sci Technol 6(3):153–160. https://doi.org/10.4236/wjnst.2016.63
Taoufiq L, Laamyem A, Boukhair A et al (2018) Radiological assessment of wastewater treatment processes based on the use of coal ashes as a flters. J Radiat Res Appl Sci 11:217–224. https://doi.org/10.1016/j.jrras.2018.01.006
Tsapalov and Kovler (2018) Control of radon emanation at determination of activity concentration index for building materials. Constr Build Mater 160:810–817. https://doi.org/10.1016/j.conbuildmat.2017.11.116
Guo T, Malone RF, Rusch KA (2001) Stabilized phosphogypsum: Class C fly ash: Portland II cement composites for potential marine applications. Environ Sci Technol 35(19):3967–3973. https://doi.org/10.1021/es010520
Fertillizers Europe (2000) Best available techniques for pollution prevention and control in the European Fertilizer Industry. Booklet No. 1 of 8. Production of ammonia
USEPA (1980) Clean lakes program guidance manual. Report No.: EPA-440/5-81-003, United States Environmental Protection Agency (USEPA), Washington, DC., USA
WHO (2017) Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. ISBN: 978-92-4-154995-0
Diwa RR, Tabora EU, Palattao BL et al (2021) Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-08142-8
Moroccan Standards (2002) Surface water quality standards. Bulletin officiel n° 5062. http://www.environnement.gov.ma/PDFs/grille_de_qualite_des_eaux_de_surface.pdf
Chusov A, Bondarenko E, Andrianova M (2014) Study of electric conductivity of urban stream water polluted with municipal effluents. Appl Mech Mater 641–642:1172–1175. https://doi.org/10.4028/www.scientific.net/AMM.641-642.1172
Cañedo-Argüelles M, Kefford BJ, Piscart C et al (2013) Salinisation of rivers: an urgent ecological issue. Environ Pollut 173:157–167. https://doi.org/10.1016/j.envpol.2012.10.011
Shariati-Rad M, Heidari S (2020) Classification and determination of total hardness of water using silver nanoparticles. Talanta 219:121297. https://doi.org/10.1016/j.talanta.2020.121297
Haneklaus N (2021) Unconventional uranium resources from phosphates. Encycl Nucl Energy. https://doi.org/10.1016/B978-0-12-819725-7.00152-5
Haneklaus N, Sun Y, Bol R et al (2017) To extract, or not to extract uranium from phosphate rock, that is the question. Environ Sci Technol 51(2):753–754. https://doi.org/10.1021/acs.est.6b05506
Beltrami D, Cote G, Mokhtari H et al (2014) Recovery of uranium from wet process phosphoric acid by solvent extraction. Chem Rev 114:12002–12023. https://doi.org/10.1021/cr5001546
Haneklaus N, Bayok A, Fedchenko V (2017) Phosphate rocks and nuclear proliferation. Sci Glob Secur 25:143–158. https://doi.org/10.1080/08929882.2017.1394061
Chernysh Y, Yakhnenko O, Chubur V, Roubík H (2021) Phosphogypsum recycling: a review of environmental issues, current trends, and prospects. Appl Sci 11:1–22. https://doi.org/10.3390/app11041575
Saadaoui E, Ghazel N, Ben Romdhane C, Massoudi N (2017) Phosphogypsum: potential uses and problems—a review. Int J Environ Stud 74:558–567. https://doi.org/10.1080/00207233.2017.1330582
Rutherford PM, Dudas MJ, Samek RA (1994) Environmental impacts of phosphogypsum. Sci Total Environ 149:1–38. https://doi.org/10.1016/0048-9697(94)90002-7
Tayibi H, Choura M, López FA (2009) Environmental impact and management of phosphogypsum. J Environ Manag 90(8):2377–2386. https://doi.org/10.1016/j.jenvman.2009.03.007
Jia G, Buchetti M, Contiet D, al, (2022) Radioecological studies of the main naturally occurring radionuclides in the area of Gela Phosphate Industry (Italy) through radioanalytical separation and measurement techniques. Appl Radiat Isot 184:110173. https://doi.org/10.1016/j.apradiso.2022.110173
Acknowledgements
The German Federal Ministry of Education and Research (Project Number: 033RU020A) provided support for this project under the coordination of the ERA-MIN3 action, which has received funding from the European Union under the Horizon 2020 Program (European Commission Grant Agreement No. 101003575). This work further received support by the Austrian Federal Ministry of Education, Science and Research (BMBWF) through Austria's Agency for Education and Internationalization (OeAD) [Grant Number: Africa UniNet P006] and the German Federal Ministry of Education and Research under Bridge2ERA2021 (Grant Number 100579052). We thank Kim Meerbach and Peter Fröhlich from TU Bergakademie Freiberg for their support provided for this research.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FEA. The first draft of the manuscript was written by FEA and MH. SO, NH, AB, MB commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arhouni, F.E., Hakkar, M., Ouakkas, S. et al. Evaluation of the physicochemical, heavy metal and radiological contamination from phosphogypsum discharges of the phosphoric acid production unit on the coast of El Jadida Province in Morocco. J Radioanal Nucl Chem 332, 4019–4028 (2023). https://doi.org/10.1007/s10967-023-09079-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09079-w