Abstract
In this paper, we have investigated 18 water samples collected from various sources, e.g., surface, underground and river water, as specimens for their sodium and potassium ions content in and around Dhanbad, a mining town, using the flame photometry. We have plotted the contour maps to show the spatial distributions of the dissolved sodium and potassium cations in the groundwater and surface water sources in and around the Dhanbad city to identify the relative contributions of human and natural phenomena to it. Along with it, water quality index (WQI) is calculated to evaluate whether the collected surface, ground and river water samples are fit for human consumption for the residents of those areas. The water of Maithan Dam has been observed to have the least sodium and potassium concentrations of 16 mg/l and 7 mg/l, respectively, which make it most suitable for human consumption. The water of Rani Talab Pond has the highest sodium and potassium contents of 49 mg/l and 24 mg/l. WQI values of all the samples are found to be less than 50, which indicates they are safe to consume by the humans. Reduction in the use of pesticides, potassium permanganate and water softeners is recommended to maintain WQI of the Dhanbad city within safe limit.
Similar content being viewed by others
Introduction
The possibility of employment of characteristic emission from excited atoms in quantitative analysis of elements for analytical chemical science was first experimentally studied by Bunsen and Kirchhoff. This very principle was actually employed for the development of instruments such as flame photometer for analytical chemical science experiments for quantitative analysis of potassium and sodium alkali metal ions. For the detection of trace metals all across the world, atomic emission is considered as an effective tool. In order to develop a robust and quantitative atomic emission-based method, there are many considerations that need to be taken into accounts such as proper selection of atomization and excitation source, proper wavelength along with slit width selection, minimization of outside chemical and spectral interference and techniques to standardization. Progress in the development of flame photometer is accelerated with the introduction of nebulizer, which enables the introduction of the sample in the form of aerosol inside the air/acetylene flame. At that time, emission was dispersed by quartz prism spectrograph, and emission was recorded photographically. As a result of this, a low-cost, highly sensitive instrument such as flame photometer was developed for the precise determination of alkali metal ions content in the samples.
Other precise techniques such as atomic absorption spectroscopy (Parker 1972) (AAS), inductively coupled plasma atomic emission spectroscopy (ICP-AES) were also developed alongside to increase the resolution of other metal ions detection. Flame photometer has a high resolution for the detection of alkali metal ions such as sodium (Na+) and potassium (K+). Later in the field of analytical spectrophotometers, the concept of the introduction of photomultiplier tube in the grating-based spectrophotometers to AAS was significant.
Flame photometer actually works by atomizing a solution sample into a flame and separates the characteristic spectra of an element along with measurement of emission. Low excitation is obtained from flame-based devices ranging from the temperature of 1000–3000 °C. Due to low excitation temperature from flame than arc flame, this method is well suited for alkali metal at best, i.e., sodium and potassium to alkali earth metals including calcium, barium, etc. The Flame photometer has unique advantages in detecting above-mentioned alkali and alkaline earth metals to a great extent over AAS and ICP-AES, which are expensive techniques. ICP-AES enjoys an overwhelming superiority in metal ions detection over flame photometry technique, but the efficiency of sodium and potassium detection by this technique is very low compared to flame photometry, which makes flame photometry technique the most suitable candidate. On the introduction of group II compounds consisting of alkaline earth and alkali metals into the flame, they dissociate into separate atoms. On dissociation, the atoms get excited to higher states. But at higher states, they are not stable, so they return back to the ground state with the emission of characteristics wavelength. As they return back to the ground state, they emit color in the range of visible spectrum. It is also worth to note that each alkali metal and alkaline earth metals have a characteristic wavelength.
Sodium is not harmful to a human being if it does not exceed the normal levels, which is sourced from drinking water and foods. But sodium intake turns into a cause of concern for those who are suffering from heart and kidney problem or insomnia. They are advised to take low sodium content food. Potassium is the sodium’s partner. Potassium stays inside the cells with cell barrier at the other side. Potassium is considered as an electrolyte which does not enjoy free circulation inside the human body. Concentration of potassium is responsible for withstanding gate pressure of sodium and the pressure generated due to water movement. Sodium/potassium pumps (Smith 1988) inside a cell are inseparable, which help our body in controlling neural transmission and contraction of muscles. Potassium level in the body is vital as it maintains a balanced water level inside the body, controls blood pressure and plays a pivotal role in neural transmission and contraction of muscle, which includes involuntary cardiac muscle. In the present work, different drinking water samples of mining area have been analyzed and discussion has been carried out. Dhanbad city has a large population as it is one of the most important coal mining belts of India. So, it is important to study the sodium and potassium content in the water as the water bodies in and around Dhanbad city are primary and secondary source of drinking water for the city population. It is important to study the amount of sodium and potassium contents in the water of the water bodies to rule out health hazards in school-going children and old-age people suffering from the kidney-related problem due to the presence of potassium and sodium in drinking water. Water quality index (WQI) of the collected water samples is calculated later to understand the compiled influence of different water quality, i.e., presence of dissolved solute, e.g., sodium and potassium in our case on the overall quality of the water. So, WQI calculation for all the water samples has been done to get the idea of quality of drinking water, i.e., whether it is fit for human consumption in reference to the presence of sodium and potassium in it.
Materials and methods
Study areas
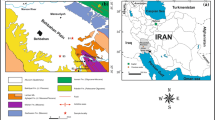
Surface water samples were collected from the various sources as shown in Fig. 1, which are situated in and around Dhanbad city of Jharkhand, India. These areas hold the highest concentration of population of Dhanbad city along with villages around Dhanbad city. So, these areas were chosen for the study. The surface waters were collected from sources, namely Baker Badh Pond, Duba Badh Pond, Kali Mandir Mandal Talab Pond, Dhaiya Basti Thakur Puli Pond and Rani Talab Pond, all of which are freshwater ponds situated in the Dhanbad city of Jharkhand, India, while one surface water sample was collected from Maithan Dam build over the flowing River Damodar. The underground waters were sourced from different places of Dhanbad city which are Hirapur, Housing Colony tube well-61/28, CSIR-CIMFR Campus bore well, Housing Colony tube well-62/28, Chandra Vihar Colony tube well, Bartand, Housing Colony bore water, Bank More tube well, Housing Colony tube well-28/413DMC (20/18/19), Steel Gate tube well, Govindpur tube well, while two underground water samples were collected from Gopalpur (Asansol)—a place which is nearly 52 km away from Dhanbad city bore water—and Chittaranjan (bore water)—a town nearly 60 km away from Dhanbad. All the above-mentioned collected water samples were examined using a flame spectrophotometer for the detection of sodium and potassium ions contained in the water samples.
Experimental procedure
Eighteen water samples were collected from various water bodies in and around Dhanbad city, of which five were surface water of ponds, while 12 were underground water comprising of four shallow and eight deep aquifers, wells, bore water (Meena and Hosamani 2004; Radha et al. 2007), tube wells along with one water sample collected from Maithan Dam built on the banks of Barakar River (biggest reservoir of Damodar Valley Corporation). For spectroscopic analysis of these water samples, a flame photometer was employed (American Public Health Association 1985). The flame photometer was calibrated using the standard stock solution of sodium and potassium having 30 ppm concentration. Various other instrumental parameters were adjusted to fine-tune the flame. After instrumental calibration, it was run to examine the collected water samples. For examination, 100 ml of water from each collected sample was taken in standard laboratory plastic bottles and then the water samples were poured inside the flame photometer. All the samples were collected during the dry winter season in the month of December when the average temperature was 20 °C. All the samples were collected once for analysis from the water sources.
Results and discussion
Analysis results as obtained from the flame photometer data are summarized below in Tables 1, 2:
The results indicate that the sodium and potassium contents in all the water samples are well within the permissible limit as per guideline laid down by World Health Organization (WHO 1984), (BIS 1991) and guidelines by other groups (Udo and Ogunwale 1978). But there are observed fluctuations in the sodium and potassium contents between different sources of water, i.e., surface water, aquifers and underground water (Maliniet al. 2003). The cause of the presence of sodium in the surface water can be attributed to time-dependent wear-out of mineral rock that contains sodium and salt deposit erosion along with infiltration of surface (Abowei 2010) water contaminated by road salt, irrigation (Erton 1950; Singh and Ram 1971; Boyd 1979; Ayers and Westcot 1985; Yadav and Khera 1993) and precipitation leaching through soils high in sodium, groundwater pollution (Ghose and Basu 1968; Turner 1981; Chatterjee 1996; Patil and Tijare 2001; Singh and Mathur 2005; Gupta and Shukla 2006) by sewage effluent (Theroux et al. 1943; Manivasakam 1996; Jakhrani 2009) and infiltration of leachate from landfills or industrial sites (Dwivedi and Pandey 2002; Chaurasia and Pandey 2007). The source of potassium in groundwater may come from hard water treatment by ion exchange treatment, or it may be sourced from accidental discharge of a large amount of potassium. Treatment of water by potassium permanganate is another major source that adds to enhanced potassium level in the water. Water softeners used to reduce the hardness of water are another way by which potassium gets introduced inside the water bodies. The sodium and potassium ions content in the analyzed samples as shown in Fig. 2a, b for collected surface and underground water give us the relative weightage of sodium and potassium ions contained in the water collected from different sources in and around the Dhanbad city.
a Spatial distribution of potassium ions (\({\mathrm{K}}^{+})\) in the pond, surface water and underground water bodies in ppm, in and around the Dhanbad district of Jharkhand represented by contour mapping, b Spatial distribution of sodium ions (\({\mathrm{N}\mathrm{a}}^{+}\)) in the pond, surface water and underground water bodies in ppm, in and around the Dhanbad district of Jharkhand represented by contour mapping
The trend observed in experimental values of sodium and potassium contents in the water of ponds, (Bronmark and Hansson 2005; Kiran 2010), surface water and underground water as given in Table 2, 3 is that underground water has a much higher content of sodium present in it compared to surface water, which may be ascribed to the fact that there is a high probability of the presence of salt rock bed very near to underground water bed, which leads to dissolving of the sodium ions with it, whereas potassium content in surface water is higher than surface water due to the human activities mentioned above.
In order to get a glimpse of variation of sodium (Rajesh and Murthy 2004) and potassium content in the entire region over which the sources of sample water are distributed will help us in understanding the interplay of role of human activity contribution to sodium, potassium ions present in water versus underground rock bed geology contribution to alkali metal ions in water. It showed that in and around the heart of Dhanbad city, sodium and potassium ions content is in the higher range, while as we move away from the city area where human habitation is negligible, a drop in potassium ion content, which is solely due to human activity, and sodium ion content in the water, which is due to the role of earth rock bed geology, is noticed (Madhuri et al. 2004; Narayan and Prasad 2006).
This trend may have arisen from the fact that in and around Dhanbad city, there exists a huge volume of a coal bed that may have inside hidden sodium rock salt bed that continuously gets mixed with the water due to the erosion of sodium rock salt bed. This explanation gets firm footing as can be seen in Fig. 2b: as we move away from Dhanbad, still we can find high content of sodium in the water bodies as the coal bed is spread across large areas away from the Dhanbad city. So, to get the insight into the quality of water qualitatively and quantitatively, water quality index (WQI) is calculated using relative weightage of parameters starting from 1 and so on based on the significance of a particular dissolved solute in the water; i.e., the most important one will get higher values like 5,6, etc., while the less relevant one will get values like 1, 2, etc., respectively.
where Ci denotes chemical parameter concentration in each and every water sample in mg/L and Si stands for standard for each parameter of chemical in mg/L as per the guidelines issued by the BIS (BIS 1991).
is the subindex of ith parameter.
is the water quality index which classifies water into five types from unsuitable for human use to excellent; the table of which is given below.
The water quality index of the water samples has been calculated for sodium and potassium, and inference has been drawn regarding whether they are fit for drinking (WHO 1984) or not in Table 4. So, water quality index gives us a tentative idea about the quality, (Rao and Devadas 2005) of the water.
Although the sodium and potassium contents in all the water samples are well within permissible limit fit for human consumption, the results hint toward the fact that human activities such as the use of too much water softeners, pesticides, deep penetration of used waste water (Foppen 2002) and application of potassium permanganate in water bodies for treatment must be controlled in and around the Dhanbad city.
Conclusions
The analysis of sodium and potassium ions present in 18 water samples collected from three types of water sources, e.g., surface, ponds and underground, in and around the Dhanbad city showed variable cation concentrations. The cation concentrations of water bodies of the Dhanbad city are within the safe limits and are fit for drinking, agricultural and industrial purposes. The water of Maithan Dam is found to have the least concentration of sodium and potassium, which is built on the banks of the River Barakar so we can say that because of being away from marine areas, the water of the rivers flowing in this region is much safer and not saline. The presence of a good concentration of sodium in underground waters taken from tube wells depicts that the aquifers are attached to the mineral bed of strata which contains a large amount of dissolved sodium. Although flame photometry is not a very reliable analytical technique for metal cations analysis, it has a high efficiency of sodium and potassium ions detection. Human activities in and around the Dhanbad city must also be kept under control to keep potassium ions content under control. Maithan water may be concluded to be the best fit for daily consumption based on sodium and potassium ions content. In a general sense, the water of all the water bodies is safe for human consumption and use from the point of view of sodium and potassium contents in it. So, the human beings should be careful before dumping toxins in the barren landscape, in water bodies such as rivers and ponds. We should minimize synthetic inputs to the entire food chain and optimize the management of waste disposal so that we leave a world that can sustain future generations to come.
References
American Public Health Association (1985) Standard methods for the examination of water and wastewater, 15th edn. Washington, DC
Abowei JFN (2010) Salinity, dissolved oxygen, pH and surface water temperature conditions in Nkoro River, Niger Delta Nigeria. Adv J Food Sci Technol 2(1):16–21
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO Irrigation and Drainage Paper No. 29. Food and Agriculture Organization of the United Nations, Rome
Boyd CE (1979) Water quality in warmwater fish ponds alabama agriculture experiment station. Auburn University, Auburn, p 359
Bronmark C, Hansson LA (2005) The biology of lakes and ponds. Oxford University Press, Oxford, p 285
BIS (1991) Drinking water specifications, Bureau of Indian Standards, IS:10500.
Chaurasia M, Pandey GC (2007) Study of physico-chemical characteristics of some water ponds of ayodhya-faizabad. Indian J Environ Protect 27(11):1019–1023
Dwivedi BK, Pandey GC (2002) Physico-chemical factors and algal diversity of two ponds, (Girija and Maqubara Pond), Faizabad. Pollut Res 21:361–370
Eaton FM (1950) Significance of bicarbonate in irrigation water. Soil Sci 69:121–133
Foppen J (2002) Impact of high-strength wastewater infiltration on groundwater quality and drinking water supply: the case of Sana'a. Yemen J Hydrol 263:198–216
Ghose BB, Basu AK (1968) Observation on estuarine pollution of the hooghly by the effluents from a chemical factory complex at Reshasa, West Bengal. Environ Health 10:209–218
Gupta S, Shukla DN (2006) Physico-chemical analysis of sewage water and its effect on seed germination and seedling growth of sesamumindicum. J Res Natl Dev 1:15–19
Jakhrani AQ (2009) Impact of wastewater effluents on physico-chemical properties of groundwater. Sindh Univ Res J Sci Ser 41:75–82
Kiran BR (2010) Physico-chemical characteristics of fish ponds of Bhadra project at Karnataka. RASĀYAN J Chem 3(4):671–676
Madhuri TU, Srinivas T, Jyothi E, Rani S (2004) Status of subsurface water quality in relation to some physico-chemical parameters-a study in Visakhapatnam. Indian J Environ Hlth 24:11–13
Malini S, Nagaiah N, Paramesh L, Venkataramaiah P, Balasubramaniam A (2003) Ground water quality around Mysore city, Karnataka. India Int J Environ Stud 60:87–98
Manivasakam N (1996) Physico-chemical examination of water, sewage and industrial effluents, 3rd edn. PragatiPrakashan, Coimbatore
MeenaKumari HR, Hosamani SP (2004) Ground water classification of bore wells of Mysore city based on salinity hazard and sodium absorption ratio. Nature Environ Pollut Technol 3:359–364
Narayana TS, Prasad BG (2006) Hydrogological study of subsurface waters. Nature Environ Pollut Technol 5:459–461
Parker RC (1972) Water Analysis by Atomic Absorption Spectroscopy. Varian Techtron, Geneva, pp 15–39
Patil DB, Tijare RV (2001) Studies on water quality of godchiroli lake. Pollut Res 20:257–259
Radha Krishnan R, Dharmaraj K, RanjithaKumari BD (2007) A comparative study on the physico-chemical and bacterial analysis of drinking borewell and sewage water in the three different places of Sivakasi. J Environ Biol 28:105–108
Rajesh R, Murthy TRS (2004) Ground water quality and its change over a decade: an analysis of a coastal urban environment from the west coast of India. Environ Geol 45:978–981
Rao NS, Devadas DJ (2005) Quality criteria for ground water use for development on an area. J Appl Geochem 7:9–24
Singh RP, Mathur P (2005) Investigation of variation in physico-chemical characteristics of a fresh water reservoir of Ajmer city, Rajasthan. Indian J Environ Sci 9:57–61
Singh S, Ram H (1971) A comparative study on pond and adjoining cultivated soils. Indian J Agric Chem 4(1):13–20
Smith KT (1988) Trace minerals in foods. CRC, Amsterdam28
Turner DR (1981) Pollution: causes, effects and control. In: Harrison RM (ed) Pollution: causes, effects and control, 2nd edn. Royal society of chemistry, London, pp 1–200
Theroux FR, Elridge EF, LeRoy MW (1943) Laboratory manual for chemical and bacterial analysis of waters and sewage. McGraw Hill Inc, London, p 274
Udo EJ, Ogunwale AJ (1978) Laboratory manual for the analysis of the soils plant and water samples. Department of Agronomy, University of Ibadan, Ibadan, pp 70–76
WHO (1984) Guidelines of drinking water quality in health criteria and other supporting information, Vol. 2, p. 336
Yadav BR, Khera MS (1993) Analysis of irrigation waters. In: Tandon HLS (ed) Methods of analysis of soils, plants waters and fertilizers. FDCO, New Delhi, p 144
Acknowledgements
Authors are grateful to Dr. P.K. Singh, Director of Central Institute of Mining and Fuel Research, Dhanbad, India, for the support and encouragement during the preparation of the paper. One of the authors (Ms. Payal Banerjee) is thankful to her Department of Applied Chemistry for their full cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banerjee, P., Prasad, B. Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl Water Sci 10, 113 (2020). https://doi.org/10.1007/s13201-020-01188-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01188-1