Abstract
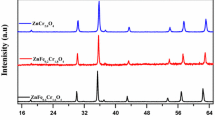
Fission isotopes contribute approximately 10% of the total radioactivity removed from the heat transport circuit of reactor systems during the chemical decontamination process. Simulated corrosion products (SCP) (CrFeO3 and MxCrFe1−xO3) and with fission isotope substituted in SCP [MxNiCrFe1−xO4 (M = La, Ce and Zr; x ≤ 0.05)] were synthesised and its dissolution behavior were studied in permanganate medium. Fission isotope metal ion substitution in SCP showed enhanced ‘Cr’ dissolution rates during the permanganate treatment. Among the fission isotope (La, Ce and Zr) substituted oxide dissolution studies, ‘La’ release was more compare to ‘Ce’ and ‘Zr’. The release behavior of fission products studies showed that dissolved lanthanum is getting sorbed onto the insitu formed MnO2.







Similar content being viewed by others
References
Allen GC, Jutson JA, Tempest PA (1988) Characterization of nickel–chromium–iron spinel-type oxides. J Nucl Mater 158:96–107. https://doi.org/10.1016/0022-3115(88)90159-6
Swan T, Segal MG, Williams WJ (1987) LOMI decontamination reagents and related oxidation process. EPRI, Calif, USA
Venkateswaran G (2000) Metal ion passivation and control of radiation field build-up. In: Venkateswaran G (ed) National symposium on water steam chemistry in nuclear power plants and industry units. BARC, Mumbai, India, pp 22–33
Comley GCW (1985) The significance of corrosion products in water reactor coolant circuits. Prog Nucl Energy 16:41–72. https://doi.org/10.1016/0149-1970(85)90005-8
Velmurugan S, Rufus AL, Sathyaseelan VS et al (2011) Experience with dilute chemical decontamination in indian pressurized heavy water reactors. Energy Procedia 7:645–649. https://doi.org/10.1016/j.egypro.2011.06.086
Merz ER (2003) Handbook of radioactive contamination and decontamination. Nucl Eng Des. https://doi.org/10.1016/0029-5493(93)90232-x
Manjanna J, Venkateswaran G, Sherigara BS, Nayak PV (2002) Synthesis and dissolution of chromium substituted magnetites in V(II)-EDTA formulation. Indian J Chem Technol 9:60–67
Manjanna J, Venkateswaran G (2002) Effect of oxidative pretreatment for the dissolution of Cr-substituted hematites/magnetites. Ind Eng Chem Res 41:3053–3063. https://doi.org/10.1021/ie010344d
Tripathi VS, Manjanna J, Venkateswaran G et al (2004) Electrolytic preparation of vanadium(II) formate in pilot-plant scale using stainless steel mesh electrodes: dissolution of α-Fe2O3/Fe1.6Cr0.4O3 in an aqueous VII-NTA complex. Ind Eng Chem Res. https://doi.org/10.1021/ie0400292
Mali A, Ataie A (2005) Structural characterization of nano-crystalline BaFe12O 19 powders synthesized by sol–gel combustion route. Scr Mater. https://doi.org/10.1016/j.scriptamat.2005.06.037
Vadivelu B, Palogi C, Madapusi SP et al (2017) Dissolution of chromite in oxidizing media and sorption of dissolved metal ion onto in situ formed manganese dioxide. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5626-y
Hasany SM, Chaudhary MH (1984) Adsorption behaviour of microamounts of cesium on manganese dioxide. J Radioanal Nucl Chem Artic. https://doi.org/10.1007/BF02036963
Valsala TP, Joseph A, Sonar NL et al (2010) Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J Nucl Mater. https://doi.org/10.1016/j.jnucmat.2010.07.017
Bhagyashree K, Kar A, Kasar S et al (2014) Sorption of americium from low-level liquid wastes by nanocrystalline MnO2. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-013-2895-y
Le NC, Van Phuc D (2015) Sorption of lead (II), cobalt (II) and copper (II) ions from aqueous solutions by γ-MnO2 nanostructure. Adv Nat Sci Nanosci Nanotechnol. https://doi.org/10.1088/2043-6262/6/2/025014
Sutka A, Mezinskis G (2012) Sol–gel auto-combustion synthesis of spinel-type ferrite nanomaterials. Front Mater Sci 6(2):128–141
Leonard M (2004) Vogel’s textbook of quantitative chemical analysis. 5th edn. Endeavour. https://doi.org/10.1016/0160-9327(90)90087-8
Segal MG, Williams WJ (1986) Kinetics of metal oxide dissolution. Oxidative dissolution of chromium(III) oxide by potassium permanganate. J Chem Soc Faraday Trans Phys Chem Condens Phases 82:3245. https://doi.org/10.1039/f19868203245
Christoffersen J, Christoffersen MR (1976) The kinetics of dissolution of calcium sulphate dihydrate in water. J Cryst Growth. https://doi.org/10.1016/0022-0248(76)90247-5
Ahmad I, Kousar R, Niazi B (2013) Structural and electrical properties of lanthanum substituted spinel ferrites. Mater Chem Phys 92(2):310–321
Sorescu M, Diamandescu L, Tomescu A, Krupa S (2009) Synthesis and sensing properties of zirconium-doped hematite nanoparticles. Phys B Condens Matter 404:2159–2165. https://doi.org/10.1016/j.physb.2009.04.006
Garnier P, Joseph V, Krachewski R (2013) Lanthanum interaction with surface preparations. ECS Trans 58:119–125. https://doi.org/10.1149/05806.0119ecst
Fendorf S, Fendorf M (1996) Sorption mechanisms of lanthanum on oxide minerals. Clays Clay Miner 44:220–227. https://doi.org/10.1346/CCMN.1996.0440207
Abellan P, Moser TH, Lucas IT et al (2017) The formation of cerium(III) hydroxide nanoparticles by a radiation mediated increase in local pH. RSC Adv 7:3831–3837. https://doi.org/10.1039/C6RA27066B
Tamilmani S, Lowalekar V, Raghavan S, Small R (2005) Dissolution characteristics of ceria in ascorbic acid solutions with implications to cleaning. Solid State Phenom 103–104:283–286. https://doi.org/10.4028/www.scientific.net/SSP.103-104.283
Brookins DG (1988) Zirconium. Eh-pH diagrams for geochemistry. Springer, Berlin, pp 116–117
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balaji, V., Chandramohan, P., Velmurugan, S. et al. Fission isotope release kinetics and its sorption on insitu formed MnO2 during the dissolution of fission isotope substituted chromium oxide in HMnO4. J Radioanal Nucl Chem 324, 791–801 (2020). https://doi.org/10.1007/s10967-020-07094-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07094-9