Abstract
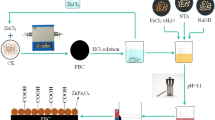
In this paper, the capture of radiocadmium (Cd(II)) by adsorption onto the titanate nanotube/iron oxide (TNT/IOM) magnetic composite as a function of contact time, pH, ionic strength, foreign cation and anion ions, humic acid (HA) and fulvic acid (FA) was studied using batch technique. The results indicated that the adsorption of Cd(II) onto the TNT/IOM magnetic composite was dependent on ionic strength at pH <9.0, but was independent of ionic strength at pH >9.0. Outer-sphere surface complexation were the main mechanism of Cd(II) adsorption onto the TNT/IOM magnetic composite at low pH values, whereas the adsorption was mainly dominated via inner-sphere surface complexation at high pH values. The adsorption of Cd(II) onto the TNT/IOM magnetic composite was dependent on foreign cation and anion ions at low pH values, but was independent of foreign cation and anion ions at high pH values. A positive effect of HA/FA on Cd(II) adsorption onto the TNT/IOM magnetic composite was found at low pH values, while a negative effect was observed at high pH values. From the results of Cd(II) removal by the TNT/IOM magnetic composite, the optimum reaction conditions can be obtained for the maximum removal of Cd(II) from water. It is clear that the best pH values of the system to remove Cd(II) from solution by using the TNT/IOM magnetic composite are 7.0–8.0. Considering the low cost and effective disposal of Cd(II)-contaminated wastewaters, the best condition for Cd(II) capture by the TNT/IOM magnetic composite is at room temperature and solid content of 0.5 g L−1. These results are quite important for estimating and optimizing the removal of Cd(II) and related metal ions by the TNT-based magnetic composite.











Similar content being viewed by others
References
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Li J, Li Y, Lu J (2009) Adsorption of herbicides 2,4-d and acetochlor on inorganic–organic bentonites. Appl Clay Sci 46:314–318
Li J, Lu J, Li Y (2009) Carboxylmethylcellulose/bentonite composite gels: water sorption behavior and controlled release of herbicide. J Appl Polym Sci 112:261–268
Li J, Jiang M, Wu H, Li Y (2009) Addition of modified bentonites in polymer gel formulation of 2,4-d for its controlled release in water and soil. J Agric Food Chem 57:2868–2874
Li J, Li Y, Dong H (2008) Controlled release of herbicide acetochlor from clay/carboxymethylcellulose gel formulations. J Agric Food Chem 56:1336–1342
Li J, Yao J, Li Y, Shao Y (2012) Controlled release and retarded leaching of pesticides by encapsulating in carboxymethyl chitosan/bentonite composite gel. J Environ Sci Health B 47:795–803
Dong H, Li J, Li Y, Hu L, Luo D (2012) Improvement of catalytic activity and stability of lipase by immobilization on organobentonite. Chem Eng J 181–182:590–596
Brennecka GA, Wasylenki LE, Bargar JR, Weyer S, Anbar AD (2011) Uranium isotope fractionation during adsorption to Mn-oxyhydroxides. Environ Sci Technol 45:1370–1375
Lafferty BJ, Ginder-Vogel M, Sparks DL (2010) Arsenite oxidation by a poorly crystalline manganese-oxide 1: stirred-flow experiments. Environ Sci Technol 44:8460–8466
Zhang Y, Li Y, Zheng X (2011) Removal of atrazine by nanoscale zero valent iron supported on organobentonite. Sci Total Environ 409:625–630
Zhang Y, Li Y, Li J, Hu L, Zheng X (2011) Enhanced removal of nitrate by a novel composite: nanoscale zero valent iron supported on pillared clay. Chem Eng J 171:526–531
Zhang Y, Li Y, Li J, Sheng G, Zhang Y, Zheng X (2012) Enhanced Cr(VI) removal by using the mixture of pillared bentonite and zero-valent iron. Chem Eng J 185–186:243–249
Li J, Li Y, Meng Q (2010) Removal of nitrate by zero-valent iron and pillared bentonite. J Hazard Mater 174:188–193
Li Y, Zhang Y, Li J, Zheng X (2011) Enhanced removal of pentachlorophenol by a novel composite: nanoscale zero valent immobilized on organobentonite. Environ Pollut 159:3744–3749
Li Y, Zhang Y, Li J, Sheng G, Zheng X (2013) Enhanced reduction of chlorophenols by nanoscale zerovalent iron supported on organobentonite. Chemosphere 92:368–374
Li Y, Li J, Zhang Y (2012) Mechanism insights into enhanced Cr(VI) removal using nanoscale zerovalent iron supported on the pillared bentonite by macroscopic and spectroscopic studies. J Hazard Mater 227–228:211–218
Bedoui K, Bekri-Abbes I, Srasra E (2008) Removal of cadmium(II) from aqueous solution using pure smectite and Lewatite S 100, the effect of time and metal concentration. Desalination 223:269–273
Guo Z, Zhao D, Li Y, Chen Z, Niu H, Xu J (2011) Solution chemistry effects on sorption behavior of 109Cd(II) on Ca–montmorillonite. J Radioanal Nucl Chem 288:829–837
Ulmanu M, Anger I, Fernández Y, Castrillón L, Marañón E (2008) Batch chromium(VI), cadmium(II) and lead(II) removal from aqueous solutions by horticultural peat. Water Air Soil Pollut 194:209–216
Wang Y, Tang XW, Chen YM, Zhan LT, Li ZZ, Tang Q (2009) Adsorption behavior and mechanism of Cd(II) on loess soil from China. J Hazard Mater 172:30–37
Palágyi Š, Salzer P, Mitro A (2006) Sorption, desorption and extraction of cadmium from some arable and forest soils. J Radioanal Nucl Chem 269:103–113
Doyurum S, Çelik A (2006) Pb(II) and Cd(II) removal from aqueous solutions by olive cake. J Hazard Mater 138:22–28
Low KS, Lee CK, Liew SC (2000) Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem 36:59–64
Lee SM, Davis AP (2001) Removal of Cu(II) and Cd(II) from aqueous solution by seafood processing waste sludge. Water Res 35:534–540
Rengan K, Sun BC (2004) Sorption of Zn(II) and Cd(II) by chelating resins: comparison of three resins. J Radioanal Nucl Chem 262:175–182
Kadirvelu K, Namasivayam C (2003) Activated carbon from coconut coir pith as metal adsorbent: adsorption of Cd(II) from aqueous solution. Adv Environ Res 7:471–478
Sheng G, Hu B Role of solution chemistry on the trapping of radionuclide Th(VI) using titanate nanotubes as an efficient adsorbent. J Radioanal Nucl Chem. doi:10.1007/s10967-012-2389-3
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1998) Formation of titanium oxide nanotube. Langmuir 14:3160–3163
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1999) Titania nanotubes prepared by chemical processing. Adv Mater 11:1307–1311
Lee CK, Lin KS, Wu CF, Lyu MD, Lo CC (2008) Effects of synthesis temperature on the microstructures and basic dyes adsorption of titanate nanotubes. J Hazard Mater 150:494–503
Lee CK, Wang CC, Juang LC, Lyu MD, Hung SH, Liu SS (2008) Effects of sodium content on the microstructures and basic dye cation exchange of titanate nanotubes. Colloids Surf A 317:164–173
An HQ, Zhu BL, Wu HY, Zhang M, Wang SR, Zhang SM, Wu SH, Huang WP (2008) Synthesis and characterization of titanate and CS2-modified titanate nanotubes as well as their adsorption capacities for heavy metal ions. Chem J Chin Univ 29:439–444
Liu SS, Lee CK, Chen HC, Wang CC, Juang LC (2009) Application of titanate nanotubes for Cu(II) ions adsorptive removal from aqueous solution. Chem Eng J 147:188–193
Sheng G, Dong H, Shen R, Li Y (2013) Microscopic insights into the temperature dependent adsorption of Eu(III) onto titanate nanotubes studied by FTIR, XPS, XAFS and batch technique. Chem Eng J 217:486–494
Sheng G, Yang S, Zhao D, Sheng J, Wang X (2012) Adsorption of Eu(III) on titanate nanotubes studied by a combination of batch and EXAFS technique. Sci China Chem 55:182–194
Sheng G, Yang S, Sheng J, Zhao D, Wang X (2011) Influence of solution chemistry on the removal of Ni(II) from aqueous solution to titanate nanotubes. Chem Eng J 168:178–182
Chen CL, Wang XK, Nagatsu M (2009) Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ Sci Technol 43:2362–2367
Li Y, Wang S, Cao A, Zhao D, Zhang X, Xu C, Luan Z, Ruan D, Liang J, Wu D, Wei B (2001) Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem Phys Lett 350:412–416
Peng X, Luan Z, Di Z, Zhang Z, Zhu C (2005) Carbon nanotubes–iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon 43:880–883
Wang W, Serp P, Kalck P, Faria JL (2005) Photocatalytic degradation of phenol on MWNT and titania composite catalysts prepared by a modified sol–gel method. Appl Catal B 56:305–312
Dong Y, Liu Z, Li Y, Chen L, Zhang Z (2012) Effect of pH, ionic strength, foreign ions, fulvic acid and temperature on 109Cd(II) sorption to γ-Al2O3. J Radioanal Nucl Chem 292:619–627
Huang Y, Wang H, Gong S (2012) Sorption behavior of hydroxyapatite for 109Cd(II) as a function of environmental conditions. J Radioanal Nucl Chem 292:545–553
Wu CH, Lin CF, Horng PY (2004) Adsorption of copper and lead ions onto regenerated sludge from a water treatment plant. J Environ Sci Health A 39:237–252
Wu CH (2007) Studies of the equilibrium and thermodynamics of the adsorption of Cu2+ onto as-produced and modified carbon nanotubes. J Colloid Interface Sci 311:338–346
Sheng GD, Shao DD, Fan QH, Xu D, Chen YX, Wang XK (2009) Effect of pH and ionic strength on sorption of Eu(III) to MX-80 bentonite: batch and XAFS study. Radiochim Acta 97:621–630
Hu B, Cheng W, Zhang H, Sheng G (2010) Sorption of radionickel to goethite: effect of water quality parameters and temperature. J Radioanal Nucl Chem 285:389–398
Hu B, Cheng W, Zhang H, Yang S (2010) Solution chemistry effects on sorption behavior of radionuclide 63Ni(II) in illite–water suspensions. J Nucl Mater 406:263–270
Sheng G, Li Y, Dong H, Shao D (2012) Environmental condition effects on radionuclide 64Cu(II) sequestration to a novel composite: polyaniline grafted multiwalled carbon nanotubes. J Radioanal Nucl Chem 293:797–806
Sheng G, Dong H, Li Y (2012) Characterization of diatomite and its application for the retention of radiocobalt: role of environmental parameters. J Environ Radioact 113:108–115
Sheng G, Shen R, Dong H, Li Y (2013) Colloidal diatomite, radionickel and humic substance interaction: a combined batch, XPS and EXAFS investigation. Environ Sci Pollut Res 20:3708–3717
Sheng G, Hu J, Jin H, Yang S, Ren X, Li J, Chen Y, Wang X (2010) Effect of humic acid, fulvic acid, pH, ionic strength and temperature on 63Ni(II) sorption to MnO2. Radiochim Acta 98:291–299
Sheng G, Sheng J, Yang S, Hu J, Wang X (2011) Behavior and mechanism of Ni(II) uptake on MnO2 by a combination of macroscopic and EXAFS investigation. J Radioanal Nucl Chem 289:129–135
Guo Z, Xu D, Zhao D, Zhang S, Xu J (2011) Influence of pH, ionic strength, foreign ions and FA on adsorption of radiocobalt on goethite. J Radioanal Nucl Chem 287:505–512
Yang ST, Li JX, Lu Y, Chen YX, Wang XK (2009) Sorption of Ni(II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl Radiat Isot 67:1600–1608
Sheng G, Yang S, Sheng J, Hu J, Tan X, Wang X (2011) Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS and EXAFS techniques. Environ Sci Technol 45:7718–7726
Wang SW, Dong YH, He ML, Chen L, Yu XJ (2009) Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl Clay Sci 43:164–171
Sheng G, Hu J, Wang X (2008) Sorption properties of Th(IV) on the raw diatomite-effects of contact time, pH, ionic strength and temperature. Appl Radiat Isot 66:1313–1320
Sheng G, Wang S, Hu J, Lu Y, Li J, Dong Y, Wang X (2009) Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloids Surf A 339:159–166
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, L., Zheng, J. & Wang, L. Fabrication of titanate nanotubes/iron oxide magnetic composite for the high efficient capture of radionuclides: a case investigation of 109Cd(II). J Radioanal Nucl Chem 298, 1947–1956 (2013). https://doi.org/10.1007/s10967-013-2598-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2598-4