Abstract
We have set out an equation for partition of 87 neutral molecules from water to o-nitrophenyl octyl ether, NPOE, an equation for partition of the 87 neutral molecules and 21 ionic species from water to NPOE, and an equation for partition of 87 neutral molecules from the gas phase to NPOE. Comparison with equations for partition into other solvents shows that, as regards partition of neutral (nonelectrolyte) compounds, NPOE would be a good model for 1,2-dichloroethane and for nitrobenzene. In terms of partition of ions and ionic species, NPOE is quite similar to 1,2-dichloroethane and not far away from other aprotic solvents such as nitrobenzene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The partition of compounds from water to organic phases is of extreme importance in extraction studies and in the purification of organic compounds. In particular, the interactions between compounds and various possible solvents for extraction is of crucial importance. We have set out equations that encode such interactions for the partition of organic compounds from water to some 50 different solvents [1,2,3,4,5,6]. Inspection of these equations then affords a simple and practical method for the choice of a solvent in order to selectively extract a given compound from a mixture. More recently, we have extended this work to include the extraction of permanent ions (K+ or Cl− for example) and of ionic species (specifically anions from the deprotonation of carboxylic acids and cations from the protonation of nitrogen bases) [7]. The number of solvents for which we have an equation for neutral molecules and ions is still quite small, although the method has been used to obtain equations for partition from water to water–ethanol mixtures [8] and to water–methanol mixtures [9] and extended into equations for permeation from saline solutions into brains [10], microsomal binding [11], artificial membrane retention factors [12], human intestinal absorption [13], partition into cerasome [14] and human skin permeability [15]. Quite recently, Davis and Di Toro [16] have examined partition of ionic species into organic solvents using quantum chemically calculated parameters.
Some time ago we examined partition of neutral molecules and ionic species from water into o-nitrophenyl octyl ether, NPOE [17], but could use only 55 out of the partition coefficients for 88 neutral compounds that were reported [18], and could use only partition coefficients for 15 ions and ionic species. Since then, we have determined descriptors for all the 88 neutral compounds, and have collected partition coefficients for 23 ions [19,20,21]. In view of the comparatively small number of solvents for which we have equations for neutral molecules and ions, we thought it useful to construct an up-to-date equation for the water–NPOE system, and then to be able to compare this system more rigorously with the other systems that we have studied. Such comparisons have in the past identified possible safe solvent alternatives to replace several of the more hazardous organic solvents used in industrial manufacturing processes, and have found organic partitioning systems that could possibly mimic some of the biological responses. An equation describing transfer of both neutral molecules and ions from water to NPOE could also be used to estimate the dissociation constants of substituted benzoic acids and substituted phenols, as well as the dissociation constants of substituted anilinium and substituted pyridinium cations, in NPOE.
2 Methodology
For partition of neutral molecules from water to another solvent we use our well-known linear free energy relationships, LFERs, Eqs. 1 and 2 [22, 23]:
In Eq. 1, the dependent variable is log10 P, where P is the water-to-solvent partition coefficient for a series of nonelectrolytes in a given water to solvent system. In Eq. 2, the dependent variable is log10 K, where K is the gas phase to solvent system partition coefficient. The independent variables are descriptors as described previously [20,21,22,23]. E is the nonelectrolyte (or solute) excess molar refractivity in units of (cm3·mol−1)/10, S is the solute dipolarity/polarizability, A and B are the overall or summation solute hydrogen bond acidity and basicity, V is the solute McGowan characteristic volume in units of (cm3·mol−1)/100, and L is log10 K16, where K16 is the gas to hexadecane partition coefficient at 298 K. The coefficients in Eq. 1 are given in Table 1.
The experimental determination of the descriptors for neutral compounds to use in Eqs. 1 and 2 has been reviewed several times [20,21,22,23,24,25]. These experimental descriptors are available both commercially [26] and in the public domain [27], and descriptors can also be calculated for nonelectrolytes [26, 27]. It is very useful if we can calculate some of the descriptors. The E-descriptor can be obtained from a refractive index at 293 K (for liquid solutes), or can be calculated from an estimated refractive index [26]. Both available software programs [26, 27] give calculated values of E. The V-descriptor can easily be calculated from its molecular formula [22, 28] and is calculated by the two software programs [26, 27]. A valuable ‘extra’ descriptor is log10 Kw where Kw is the gas-to-water partition coefficient at 298 K; note that Kw is dimensionless. Descriptors for the 88 non-electrolytes are in Table 2, together with values of the water–NPOE partition coefficient [18], as log10 Pnpoe, values of log10 Kw and corresponding values of log10 Knpoe obtained through Eq. 3.
The determination of equation coefficients in Eq. 1 is a prerequisite for obtaining the corresponding equation for ion transfer, because we have deliberately used Eq. 1 as part of our equation for ion transfer, Eq. 4. In this equation, the coefficients c, e, s, a, b and v are set equal to the coefficients in Eq. 1 for the corresponding equation for nonelectrolytes. The descriptors J+ and J– and the equation coefficients j+ and j– refer to cations and anions. For anions j+ = 0, for cations j– = 0 and for nonelectrolytes j+ = j– = 0, and Eq. 4 then reverts to Eq. 1. The j+ and j– coefficients in Eq. 4 are given in Table 1.
Ionic partition coefficients from water to NPOE, or their equivalent as Gibbs energies of transfer, have been determined by a number of workers [19,20,21]. As we have previously pointed out, experimental values can only be obtained for neutral combinations of ions, e.g. (K+ + Cl–), and single-ion values have to be referred to some particular convention. Usually the TATB convention [29,30,31] is used, with log10 P(Ph4P+) or log10 P(Ph4As+) = log10 P (Ph4B–). The various conventions that have been put forward in order to obtain single-ion values have been evaluated [30, 31] and the TATB convention selected as the recommended one. All our studies have used this convention, and this is the convention that Wilke and Zerihun [19] and Samec et al. [21] have used. Gulabowski et al. [20] in their determination of partition coefficients of anions used the decamethylferrocene/decamethylferrocinium couple as a standard, and so for consistency all their ionic partition coefficients had to be converted to the TATB convention. The descriptors for the ions [7] are in Table 3, and the data on ionic partition coefficients [19,20,21] are in Table 4.
3 Results
All the data that we need to construct Eq. 1 for the water-to-NPOE system are in Table 2. One solute, 4-bromobenzoic acid, was an outlier, and for the remaining 87 solutes we obtained Eq. 5:
The outlier, 4-bromobenzoic acid, had an observed value of log10 Pnpoe as 0.82; observed values for 4-chlorobenzoic acid and 4-iodobenzoic acid are 0.88 and 1.46, and so the observed value for 4-bromobenzoic acid does seem to be out of line. In Eq. 5, N is the number of solutes, SD is the regression standard deviation, R is the correlation coefficient, F is the F-statistic, PRESS and Q2 are the leave-one-out statistics and PSD is the predictive standard deviation [32].
Values of the gas to NPOE partition coefficient were obtained through Eq. 3 and are listed in Table 2. Application of the LFER Eq. 2 leads to Eq. 6. As before, the solute 4-bromobenzoic acid was left out.
Details of observed values of log10 Knpoe for ions and ionic species are in Table 4. There are a number of discrepancies between the sets of data [19, 21], and so we took the values of Wilke and Zerihun [19] for consistency, and supplemented these with data on anions from Gulabowski et al. [20]. In Eq. 4 the coefficients c, e, s, a, b and v are taken as the same as those for the equation for neutral species, Eq. 5, and so there are only two coefficients to be determined. The full equation is shown as Eq. 7:
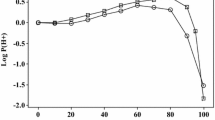
The usual statistics, as in Eqs. 5 and 6, do not apply to Eq. 7 because the coefficients c, e, s, a, b and v are fixed. However, for the 21 ions, Eq. 7 fits values of log10 Pnpoe with an SD of only 0.236 log10 units. The observed values that we used and the calculated values are in Table 4. We left out data on the bromide anion, the perchlorate anion and the benzoate anion, that were very considerably out of line. It is perhaps not surprising that we find a number of values of log10 Pnpoe for ions and ionic species to be out of line, considering the differences in some of the experimental values: 0.60 for Cs+ and 0.50 for Me4N+ (Table 4). The calculated values of log10 Pnpoe for the ions show no systematic deviations, as can be seen from Fig. 1.
4 Discussion
The equations for log10 Pnpoe and log10 Knpoe for 87 neutral species are consistent with previous such equations for partition from water and the gas phase to solvents. The SD values in both Eqs. 5 and 6 are rather larger than those we usually find, but the 87 compounds include a large number of drugs for which it is more difficult to obtain descriptors. The values of the coefficients in Eqs. 5 and 6 are not particularly unusual, although the b-coefficient in Eq. 6 (0.967) indicates that NPOE has some hydrogen bond acidity. This can hardly be due to the presence of water in water-saturated NPOE because the solubility of water in NPOE is only 0.046 mol·dm−3 [21], less than the water solubility in nitrobenzene and in 1,2-dichloroethane.
Liu et al. [18] suggested that NPOE could replace 1,2-dichloroethane as regards solvation and partition of nonelectrolytes. A simple way of analyzing the coefficients in Eq. 1 is to regard the coefficients e, s, a, b and v as points in five-dimensional space. Then the distance, D’, between a point for a particular system and the point for the NPOE system will indicate how near the particular system is to the NPOE system in terms of solubility related properties [33]. Results in terms of the distance parameter D’ [33] are in Table 5, in order of the value of D’. We give also in Table 5, details of the nature of the various water–solvent systems. For many of the water–aprotic solvent systems solubilities in the dry solvent are effectively the same as those in the solvent equilibrated with water; we denote these as w/d. The solvents for which data were obtained only using the dry solvent are denoted as ‘d’. Wet octanol is an exception in that the solubility properties of water-saturated octanol, ‘w’, are not the same as those of dry octanol. The solvent systems with nitrobenzene or 1,2-dichloroethane are chemically the closest to the NPOE system. Abraham and Martins [33] suggested that for two systems to be regarded as similar the distance parameter should not be larger than about 0.5–0.8 units. The D’ values for nitrobenzene and 1,2-dichloroethane are slightly outside this criterion, at 0.981 and 0.999 respectively, but the water–NPOE system is still closer to the nitrobenzene or 1,2-dichloroethane systems than to any of the other systems in Table 5.
We can also calculate the D’ parameter using the coefficients e, s, a, b and l from Eq. 2. These are also given in Table 5. Note that the entries are in order of the solvents—this is not quite the same as the order of D’ from Eq. 1, although the order in terms of Eq. 2 follows very closely the order in terms of Eq. 1. Again, NPOE is a better model for nitrobenzene and 1,2-dichloroethane than any of the other solvents listed, as regards nonelectrolytes.
In order to analyze partition of ionic species, we could calculate D’ using the coefficients in Eq. 4. However, the results would be dominated by the ‘nonelectrolyte’ coefficients e, s, a, and b and would yield little direct information on partition of ionic species. A direct method of assessing the various water–solvent systems in terms of ionic species is simply to survey the actual ionic partition coefficients from water to the various solvents. Unfortunately, there are very few ionic species for which partition coefficients are known across any reasonable number of systems. We can circumvent this difficulty by using the data in Tables 1 and 3 to calculate values of ionic partition coefficients for a number of representative systems as shown in Table 6. We also give the SD for the values of log10 P against the NPOE system as the standard. From the SD values, it can be seen that NPOE would be a good substitute for 1,2-dichloroethane and possibly for nitrobenzene as regards partition of ionic species. This is a quite important result. Seip et al. [34] have set out a classification of solvents in terms of their suitability as supported liquid membranes in electromembrane extraction. NPOE was rated in the highest category, whereas nitrobenzene was classed as unsuitable.
Davis and Di Toro [16] have approached the problem of descriptors for ionic species rather differently from the methods we have employed. They use quantum calculated partitions into a large number of solvents and define ionic species in terms of five descriptors only. However, they then require different equations for partition of neutral molecules, anions and cations from water into a given solvent. Davis and Di Toro [16] set out equations for the partition of anions into propanone, acetonitrile, methanol and dimethylsulfoxide with an RMSE in log10 P from 0.39 to 0.51, and an equation for partition of cations from water to octanol with an SD of 1.16 in log10 P. At the moment the two methods are independent of each other, although it would be useful if descriptors for ionic species could somehow be interchanged.
5 Conclusions
We have constructed LFERs for partition from water to NPOE and from the gas phase to NPOE for 87 neutral solutes. The latter equation is new and has not been set out before. The equations reveal that the solution properties of NPOE for nonelectrolyte solutes are quite similar to solution properties of the typical aprotic solvents 1,2-dichloroethane and nitrobenzene. Almost the same result is obtained by the examination of partition coefficients for ions and ionic species. The solution properties of NPOE for electrolytes are quite close to those of 1,2-dichloroethane although a little way away from aprotic solvents such as nitrobenzene and benzonitrile.
References
Abraham, M.H., Smith, R.E., Luchtefeld, R., Boorem, A.J., Luo, R., Acree Jr., W.E.: Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99, 1500–1515 (2010)
Stephens, T.W., Quay, A.N., Chou, V., Loera, M., Shen, C., Wilson, A., Acree Jr., W.E., Abraham, M.H.: Correlation of solute transfer into alkane solvents from water and from the gas phase with updated Abraham model equations. Glob. J. Phys. Chem. 3, 1–42 (2012)
Abraham, M.H., Acree Jr., W.E.: Descriptors for the prediction of partition coefficients and solubilities of organophosphorus compounds. Sep. Sci. Technol. 48, 884–897 (2013)
Brumfield, M., Wadawadigi, A., Kuprasertkul, N., Mehta, S., Acree Jr., W.E., Abraham, M.H.: Abraham model correlations for solute transfer into tributyl phosphate from both water and the gas phase. Phys. Chem. Liq. 53, 1–24 (2015)
Abraham, M.H., Acree Jr., W.E.: Equations for water–triolein partition coefficients for neutral species; comparison with other water–solvent partitions and environmental and toxicological processes. Chemosphere 154, 48–54 (2016)
Abraham, M.H., Acree Jr., W.E.: Gas–solvent and water–solvent partition of trans-stilbene at 298 K. J. Mol. Liquids 238, 58–61 (2017)
Abraham, M.H., Acree Jr., W.E.: Descriptors for ions and ion-pairs for use in linear free energy relationships. J. Chromatogr. A 430, 2–14 (2016)
Abraham, M.H., Acree Jr., W.E.: Equations for the partition of neutral molecules, ions and ionic species from water to water–ethanol mixtures. J. Solution Chem. 41, 730–740 (2012)
Abraham, M.H., Acree Jr., W.E.: Equations for the partition of neutral molecules, ions and ionic species from water to water–methanol mixtures. J. Solution Chem. 45, 861–874 (2016)
Abraham, M.H.: The permeation of neutral molecules, ions and ionic species through membranes: brain permeation as an example. J. Pharm. Sci. 100, 1690–1701 (2011)
Abraham, M.H., Austin, R.P.: The effect of ionized species on microsomal binding. Eur. J. Med. Chem. 47, 202–205 (2012)
Abraham, M.H., Acree Jr., W.E., Fahr, A., Lui, X.: Analysis of immobilized artificial membrane retention factors for both neutral and ionic species. J. Chromatogr. A 1298, 46–49 (2013)
Abraham, M.H.: Human intestinal absorption—neutral molecules and ionic species. J. Pharm. Sci. 103, 1956–1966 (2014)
Zhang, K., Fahr, A., Abraham, M.H., Acree Jr., W.E., Tobin, D.J., Liu, X.: Comparison of lipid membrane–water partitioning with various organic solvent–water partitions of neutral species and ionic species: uniqueness of cerasome as a model for the stratum corneum in partition processes. Int. J. Pharmaceutics 494, 1–8 (2015)
Zhang, K., Abraham, M.H., Liu, X.: An equation for the prediction of human skin permeability of neutral molecules, ions and ionic species. Int. J. Pharmaceutics 521, 259–266 (2017)
Davis, C.W., Di Toro, D.M.: Predicting solvent–water partitioning of charged organic species using quantum-chemically estimated Abraham pp-LFER solute parameters. Chemosphere 164, 632–634 (2016)
Abraham, M.H., Zhao, Y.H.: Characterisation of the water/o-nitrophenyl octyl ether system in terms of the partition of nonelectrolytes and ions. Phys. Chem. Chem. Phys. 7, 2418–2422 (2005)
Liu, X., Bouchard, G., Muller, N., Galland, A., Girault, H., Testa, B., Carrupt, P.-A.: Solvatochromic analysis of partition coefficients in the o-nitrophenyl octyl ether (o-NPOE)/water system. Helv. Chim. Acta 86, 3533–3547 (2003)
Wilke, S., Zerihun, T.: Standard Gibbs energies of ion transfer across the water/2-nitrophenyl octyl ether interface. J. Electroanal. Chem. 515, 52–60 (2001)
Gulaboski, R., Galland, A., Bouchard, G., Caban, K., Kretschmer, A., Carrupt, P.-A., Stojek, Z., Girault, H.H., Scholz, F.: A comparison of solvation properties of 2-nitrophenyloctyl ether, nitrobenzene, and n-octanol as assessed by ion transfer experiments. J. Phys. Chem. B 108, 4565–4572 (2004)
Samec, Z., Langmaier, J., Trojanek, A.: Polarisation phenomena at the water/o-nitrophenyl octyl ether interface. Part 1. Evaluation of the standard Gibbs energies of ion transfer from the solubility and voltammetric measurements. J. Electroanal. Chem. 409, 1–7 (1996)
Abraham, M.H.: Scales of hydrogen bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73–83 (1993)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: The determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037, 29–47 (2004)
Poole, C.F., Atapattu, S.N., Poole, S.K., Bell, A.K.: Determination of solute descriptors by chromatographic methods. Anal. Chim. Acta 652, 32–53 (2009)
Clarke, E.D., Mallon, L.: The Determination of Abraham descriptors and their application to crop protection research. In: Jeschke, P., Kramer, W., Schirmer, U., Witschel, M. (eds.) Modern Methods in Crop Protection Research. Wiley-VCH Verlag GmbH & Co., Weinheim (2012)
Absolv: ADME Suite 5.0, Advanced Chemistry Development, Toronto
Endo, S., Brown, T.N., Watanabe, N., Ulrich, N., Bronner, G., Abraham, M.H., Goss, K-U.: UFZ-LSER database v 3.1 [Internet]. Helmholtz Centre for Environmental Research-UFZ, Leipzig (2015). www.ufz.de/lserd
Abraham, M.H., McGowan, J.C.: The use of characteristic volumes to measure cavity terms in reversed-phase liquid chromatography. Chromatographia 23, 243–246 (1987)
Grunwald, E., Baughman, G., Kohnstam, C.: The solvation of electrolytes in dioxane–water mixtures, as deduced from the effect of solvent change on the standard partial molar free energy. J. Am. Chem. Soc. 82, 5801–5811 (1960)
Popovych, O., Bates, R.G.: Estimation of medium effects for single ions in non-aqueous solvents. Crit. Rev. Anal. Chem. 1, 73–117 (1970)
Cox, B.G., Parker, A.J.: Solvation of ions. XVII. Free energies, heats, and entropies of transfer of single ions from protic to dipolar aprotic solvents. J. Am. Chem. Soc. 95, 402–407 (1973)
Abraham, M.H., Acree Jr., W.E., Leo, A.J., Hoekman, D.: The partition of compounds from water and from air into wet and dry ketones. New J. Chem. 33, 568–573 (2009)
Abraham, M.H., Martins, F.: Human skin permeation and partition: general linear free-energy relationship analyses. J. Pharm. Sci. 93, 1508–1523 (2004)
Seip, K.F., Faizi, F., Vergel, C., Gjelstad, A., Pedersen-Bjergaard, S.: Stability and efficiency of supported liquid membranes in electromembrane extraction—a link to solvent properties. Anal. Bioanal. Chem. 406, 2151–2161 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abraham, M.H., Acree, W.E. & Liu, X. Partition of Neutral Molecules and Ions from Water to o-Nitrophenyl Octyl Ether and of Neutral Molecules from the Gas Phase to o-Nitrophenyl Octyl Ether. J Solution Chem 47, 293–307 (2018). https://doi.org/10.1007/s10953-018-0717-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0717-0