Abstract
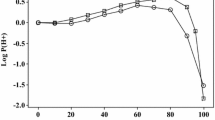
We use literature data on solubilities of 46 compounds in the water–isopropanol (IPA) system to obtain the corresponding partition coefficients, P, for transfer from water to water–IPA mixtures. We have then used our previously constructed linear free energy equation to obtain equations that correlate log10 P at water–IPA intervals across the entire water–IPA system. These equations can then be used to predict partition coefficients and solubilities of further compounds in the water–IPA systems at 298 K. The coefficients in our linear free energy equation encode information on the physicochemical properties of the water–IPA mixtures. We show that the hydrogen bond basicity of the water–IPA mixtures only increases slightly from water to IPA, but that the hydrogen bond acidity of the mixtures decreases markedly from water to IPA in a smooth continuous manner. We have also used data on ions and on ionic species to set out equations for the estimation of their partition coefficients from water to water–IPA mixtures. We find that for partition from water to IPA itself, log10 P = − 1.81 for H+.



Similar content being viewed by others
References
Jouyban, A., Chew, N.Y.K., Chan, H.K., Sabour, M., Acree, W.E.: A unified cosolvency model for calculating solute solubility in mixed solvents. Chem. Pharm. Bull. 53, 634–637 (2005)
Jouyban, A., Acree, W.E., Jr.: Mathematical derivation of the Jouyban-Acree model to represent solute solubility data in mixed solvents at various temperatures. J. Mol. Liq. 256, 541–547 (2018)
Kamlet, M.J., Abboud, J.L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. 23. A comprehensive collection of solvatochromic parameters π*, α and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 48, 2877–2887 (1983)
Taft, R.W., Abboud, J.-L.M., Kamlet, M.J., Abraham, M.H.: Linear solvation energy relations. J. Solution Chem. 14, 153–186 (1985)
Abraham, M.H., Acree, W.E., Jr.: Partition coefficients and solubilities of compounds in the water–ethanol solvent system. J. Solution Chem. 40, 1279–1290 (2011)
Abraham, M.H., Acree, W.E., Jr.: Equations for the partition of neutral molecules, ions and ionic species from water to water–ethanol mixtures. J. Solution Chem. 41, 730–740 (2012)
Abraham, M.H., Acree, W.E., Jr.: Equations for the partition of neutral molecules, ions and ionic species from water to water–methanol mixtures. J. Solution Chem. 45, 861–874 (2016)
Abraham, M.H.: Scales of hydrogen bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73–83 (1993)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: The determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037, 29–47 (2004)
Acree, W.E., Jr., Abraham, M.H.: Solubility predictions for crystalline nonelectrolyte solutes dissolved in organic solvents based upon the Abraham general solvation model. Can. J. Chem. 79, 1466–1476 (2001)
Stovall, D.M., Givens, C., Keown, S., Hoover, K.R., Rodriguez, E., Acree, W.E., Jr., Abraham, M.H.: Solubility of crystalline nonelectrolyte solutes in organic solvents: mathematical correlation of ibuprofen solubilities with the Abraham solvation parameter model. Phys. Chem. Liq. 43, 261–268 (2005)
Abraham, M.H., Smith, R.E., Luchtefeld, R., Boorem, A.J., Luo, R., Acree, W.E., Jr.: Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99, 1500–1515 (2010)
Abraham, M.H., McGowan, J.C.: The use of characteristic volumes to measure cavity terms in reversed-phase liquid chromatography. Chromatographia 23, 243–246 (1987)
Poole, C.F., Atapattu, S.N., Poole, S.K., Bell, A.K.: Determination of solute descriptors by chromatographic methods. Anal. Chim. Acta 652, 32–53 (2009)
Poole, C.F., Ariyasena, T.C., Lenca, N.: Estimation of the environmental properties of compounds from chromatographic measurements and the solvation parameter model. J. Chromatogr. A. 1317, 85–104 (2013)
Clarke, E. D., Mallon, L.: The Determination of Abraham descriptors and their application to crop protection research. In: Jeschke, P., Kramer, W., Schirmer, U., Witschel, M. (eds.) Modern Methods in Crop Protection Research,Wiley–VCH Verlag GmbH & Co. (2012)
Fakhree, M.A.A., Ahmadian, S., Panahi-Azar, V., Acree, W.E., Jr., Jouyban, A.: Solubility of 2-hydroxybenzoic acid in water, 1-propanol, 2-propanol and 2- propanone at (298.2 to 338.2) K and their aqueous binary mixtures at 298 K. J. Chem. Eng. Data 57, 3303–3307 (2012)
Cheong, W.J., Carr, P.W.: Limiting activity coefficients and gas–liquid partition coefficients of alkylbenzenes in hydro-organic solvents. J. Chromatogr. 500, 215–239 (1990)
Wang, S., Chen, D.: Solubility of piperonal in different pure solvents and binary isopropanol–water solvent mixtures. Korean J. Chem. Eng. 23, 1034–1036 (2006)
Yamamoto, H., Ichikawa, K., Tokunaga, J.: Solubility of helium in methanol + water, ethanol + water, 1-propanol + water and 2-propanol + water solutions at 25 °C. J. Chem. Eng. Data 39, 155–157 (1994)
Mohammadzade, M., Barzegar-Jalali, M., Jouyban, A.: Solubility of naproxen in 2- propanol + water mixtures at various temperatures. J. Mol. Liq. 206, 110–113 (2015)
Dali, I., Aydi, A., Alberto, C.C., Wust, Z.A., Manef, A.: Correlation and semi- empirical modeling of solubility of gallic acid in different pure solvents and in binary mixtures of propan-1-ol + water, propan-2-ol + water and acetonitrile + water from (2932 to 3182) K. J. Mol. Liq. 222, 503–519 (2016)
Chen, S.-N., Xia, Q., Lu, L.-F., Zhang, M.-S., Chen, Y.-S., Zhang, F.-B., Zhang, G.-L.: Measurement and correlation of solubilities of decanedioic acid in C4–C6 alcohol solvents. J. Chem. Eng. Data 55, 1411–1415 (2010)
Sada, E., Kito, S., Ito, Y.: Solubility of nitrous oxide in the mixtures of alcohols and water: comparison with Pierotti’s gas solubility theory. Ind. Eng. Chem. Fundam. 14, 232–236 (1975)
Li, K., Du, S., Wu, S., Cai, D., Wang, J., Zhang, D., Zhao, K., Yang, P., Yu, B., Guo, B., Li, D., Gong, J.: Determination and correlation of solubility and solution thermodynamics of oxiracetam in three (alcohol + water) binary solvents. J. Chem. Thermodyn. 96, 12–23 (2016)
Das, K., Das, A.K., Bose, K., Kundu, K.K.: Thermodynamics of transfer of benzoic acid from water to aqueous mixtures of urea and of some alcohols. J. Phys. Chem. 82, 1242–1245 (1978)
Mandal, K.R., Lahiri, S.C.: Studies on the solubilities and dissociation constants of substituted benzoic acids in 2-propanol–water mixtures and ion–solvent interactions. J. Indian Chem. Soc. 85, 901–910 (2008)
Zhu, C., Farajtabar, A., Wu, J., Zhao, H.: 5,7-Dibromo-8-hydroxyquinoline dissolved in binary aqueous co-solvent mixtures of isopropanol, N, N-dimethylformamide, 1,4- dioxane and N-methyl-2-pyrrolidone: solubility modeling, solvent effect and preferential solvation. J. Chem. Thermodyn. 148, 106138 (2020)
Zhou, Y., Xu, R., Wang, J., Zhao, H.: Equilibrium solubility and preferential solvation of 2,6-dichloro-4-nitro aniline dissolved in four aqueous mixtures of isopropanol, acetonitrile, n-propanol and N-methyl-2-pyrrolidone. J. Chem. Eng. Data 65, 2912–2920 (2020)
Li, H., Xie, Y., Li, Z., Zhao, H.: 2-Methoxy-4-nitroaniline solubility in several aqueous solvent mixtures: determination, modeling and preferential solvation. J. Chem. Eng. Data 65, 2673–2682 (2020)
Li, W., Xing, R., Zhu, Y., Zhao, H.: Lv, R,: Risperidone (I) in some aqueous mixtures of low alcohols: solubility, preferential solvation and solvent effect analysis. J. Chem. Thermodyn. 148, 106137 (2020)
Li, X., Zhu, Y., Zhang, X., Farajtabar, A., Zhao, H.: Solubility, preferential solvation, and solvent effect of microflavin in aqueous mixtures of dimethylsulfoxide, isopropanol, propylene glycol and ethanol. J. Chem. Eng. Data 65, 1976–1985 (2020)
Li, W., Farajtabar, A., Xing, R., Zhu, Y., Zhao, H., Lv: Thermodynamic solubility, solvent effect and preferential solvation analysis of rebamipide in aqueous co-solvent mixtures of propylene glycol, n-propanol, isopropanol and ethanol. J. Chem. Thermodyn. 143, 106045 (2020)
Xhou, Y., Wu, J., Farajtabar, A., Wang, J., Zhao: Solubility of monobenzone inaqueous co-solvent mixtures of several alcohols: determination, modelling and thermodynamic aspects analysis. J. Chem. Thermodyn. 142, 106023 (2020)
Li, X., He, Y., Xu, Y., Zhang, X., Zheng, M., Zhao, H.: 5-Nitrosalicylaldehyde in aqueous co-solvent mixtures of methanol, ethanol, isopropanol and acetonitrile: solubility determination, solvent effect and preferential solvation analysis. J. Chem. Thermodyn. 142, 106014 (2020)
Chen, X., Farajtabar, A., Jia, W., Zhao, H.: Solvent effect on solubility and preferential solvation analysis of buprofezin dissolved in aqueous co-solvent mixtures of N, N-dimethylformamide, ethanol, acetonitrile and isopropanol. J. Chem. Thermodyn. 138, 179–188 (2019)
Zheng, M., Farajtabar, A., Zhao, H.: Solubility of 4-amino-2, 6-dimethoxypyrimidine in aqueous co-solvent mixtures revisited: solvent effect, transfer property and preferential solvation analysis. J. Mol. Liq. 288, 111033 (2019)
Zheng, M., Xu, R., Zhao, H.: Equilibrium solubility determination, modelling and thermodynamic aspects of 6-chloroguanine in aqueous co-solvent mixtures of N, N-dimethylformamide, isopropanol, 1,4-dioxane and dimethylsulphoxide. J. Chem. Thermodyn. 134, 52–60 (2019)
Liang, J., Ma, J., Han, J., Zheng, M., Zhao, H.: Solubility determination, modeling, and preferential solvation of terephthalaldehyde acid dissolved in aqueous solvent mixtures of methanol, ethanol, isopropanol and N-methyl-2-pyrrolidone. J. Chem. Eng. Data 64, 1791–1801 (2019)
Li, X., Liu, Y., Zheng, N., Zhang, N., Farajtabar, A., Zhao, H.: Solubility modelling, solvent effect and preferential solvation of allopurinol in aqueous co-solvent mixtures of ethanol, isopropanol, N, N-dimethylfomamide and 1-methyl-2-pyrrolidone. J. Chem. Thermodyn. 131, 478–488 (2019)
Zadaliasghar, S., Jouyban, A., Martinez, F., Barzegar-Jalali, M., Rahimpour, E.: Solubility of ketoconazole in the binary mixtures of 2-propanol and water. J. Mol. Liq. 300, 112259 (2020)
Rezaei, H., Jouyban, A., Barzegar-Jalali, M., Martinez, F., Rahimpour, E.: Solubility of lamotrigine in 2-propanol + water mixtures at T = (293.2 to 313.2) K. J. Mol. Liq. 278, 592–599 (2019)
Jouyban, A., Nozohouri, S., Martinez, F.: Solubility of celecoxib in 2-propanol (1) + water (2) mixtures at various temperatures: experimental data and thermodynamic analysis. J. Mol. Liq. 254, 1–7 (2018)
Wie, N., Shang, Z., Ma, Y., Zhang, N., Gong, J., Tang, W.: Experimental determination and prediction of the solubility of alpha-(trichloromethyl) benzyl acetate in monosolvents and binary mixed solvents. J. Mol. Liq. 294, 111633 (2019)
Wie, N., Shang, Z., Zhang, N., Wang, J.: Wu, S,: Deep analysis of the solubility behavior mechanism of alpha-(trichloromethyl) benzyl acetate in three binary aqueous solvents. J. Chem. Thermodyn. 151, 106246 (2020)
Li, C., Xu, Q., Chen, X., Li, R.: Bezafibrate in several aqueous mixtures of low alcohols: solubility, solvent effect and preferential solvation. J. Chem. Thermodyn. https://doi.org/https://doi.org/10.1016/j.jct.2020.106208
Li, H., Farajtabar, A., Xie, Y., Li, Z., Zhao, H.: Apixaban (I) in several aqueous co-solvent mixtures: solubility, solvent effect and preferential solvation. J. Chem. Thermodyn. 150, 106200 (2020)
Zhang, J., Song, X., Xu.: Solubility determination and modeling for milrinone in binary solvent mixtures of ethanol, isopropanol, ethylene glycol, and N,N-dimethylformamide and water. J. Chem. Eng. Data 65, 4100–4107 (2020)
Qui, J., Huang, H., He, H., Liu, H., Hu, S., Han, J., Yi, D., An, M., Guo, Y., Wang, P.: Solubility determination and thermodynamic modeling of edaravone in different solvent systems and the solvent effect in pure solvents. J. Chem. Eng. Data 65, 3240–3251 (2020)
Chen, X., Xu, Q., Liu, Z., Zhu, X., Zheng, H., Zhao, J., Li, R.: Solubility determination, model correlation, and solvent effect analysis of nisoldepine in different solvent systems at a series of temperatures. J. Chem. Eng. Data 65, 1627–1635 (2020)
Zhang, J., Huang, C., Xu, R.: Solubility of bifonazole in four binary solvent mixtures: experimental measurement and thermodynamic modeling. J. Chem. Eng. Data 64, 2641–2648 (2019)
Wu, J., Xu, R., Yuan, X., Zhao, J., Wang, J.: Equilibrium solubility of dinitolmide in several neat solvents and binary aqueous co-solvent mixtures: experimental determination and thermodynamic analysis. J. Chem. Thermodyn. 132, 373–382 (2019)
Gong, T., Han, D., Chen, Y., Wang, S., Tang: Solubility and data correlation of isoniazid in different pure and binary mixed solvent systems from 28315 K to 32315 K. J. Chem. Eng. Data 63, 4767–4778 (2018)
Li, Y., Li, C., Gao, X., Lv, H.: Equilibrium solubility, preferential solvation and solvent effect study of clotrimazole in several aqueous co-solvent solutions. J. Chem. Thermodyn. 151, 106255 (2020)
Abraham, M.H., Acree, W.E., Jr.: Equations for the transfer of neutral molecules and ionic species from water to organic phases. J. Org. Chem. 75, 1006–1015 (2010)
Abraham, M.H., Acree, W.E., Jr.: Descriptors for ions and ion-pairs for use in linear free energy relationships. J. Chromatogr. A 1430, 2–14 (2016)
Abraham, M.H., Acree, W.E., Jr.: The transfer of neutral molecules, ions and ionic species from water to ethylene glycol and to propylene carbonate; descriptors for pyridinium cations. New J. Chem. 34, 2298–2305 (2010)
Abraham, M.H., Acree, W.E., Jr.: The transfer of neutral molecules, ions and ionic species from water to wet octanol. Phys. Chem. Chem. Phys. 12, 13182–13188 (2010)
Stephens, T.W., De.La Rosa, N.E., Saifullah, M., Ye, S., Chou, V., Quay, A.N., Acree, W.E., Jr., Abraham, M.H.: Abraham model correlations for transfer of neutral molecules and ions to sulfolane. Fluid Phase Equilib. 309, 30–35 (2011)
Abraham, M.H., Acree, W.E., Jr.: The transfer of neutral molecules, ions and ionic species from water to benzonitrile; comparison with nitrobenzene. Thermochim. Acta 526, 22–28 (2011)
Saifullah, M., Ye, S., Grubbs, L.M., De La Rosa, N.E., Acree, W.E., Jr., Abraham, M.H.: Abraham model correlations for the transfer of neutral molecules to tetrahydrofuran and to 1,4-dioxane and for transfer of ions to tetrahydrofuran. J. Solution Chem. 40, 2082–2094 (2011)
Marcus, Y.: Thermodynamic functions of transfer of single ions from water to nonaqueous solvents and mixed solvents: Part 1 – Gibbs free energies of transfer to nonaqueous solvents. Pure Appl. Chem. 55, 97–1021 (1983)
Kalidas, C., Hefter, G., Marcus, Y.: Gibbs energies of transfer of cations from water to mixed aqueous organic solvents. Chem. Rev. 100, 819–852 (2000)
Marcus, Y.: Gibbs energies of transfer of anions from water to mixed aqueous organic solvents. Chem. Rev. 107, 3880–3897 (2007)
Chantooni, M.K., Kolthoff, I.M.: Proton solvation in the lower aliphatic alcohols with emphasis on isopropyl and tert-butyl alcohols. J. Phys. Chem. 82, 994–1000 (1978)
Chantooni, M.K., Kolthoff, I.M.: Resolution of acid strength in tert-butyl alcohol and isopropyl alcohol of substituted benzoic acids, phenols and aliphatic carboxylic acids. Anal. Chem. 51, 133–140 (1979)
Bosch, E., Ràfols, C., Rosés, M.: Ionic equilibria in neutral amphiprotic solvents: structural effects on dissociation constants of several substituted phenols and mercaptopyridines in isopropyl alcohol. Talanta 36, 1227–1231 (1989)
Ràfols, C., Rosés, M., Bosch, E.: Dissociation constants of several non-steroidal anti- inflammatory drugs in isopropyl alcohol/water mixtures. Anal. Chim. Acta 350, 249–255 (1997)
Ràfols, C., Rosés, M., Bosch, E.: A comparison between different approaches to estimate the aqueous pKa values of several non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 338, 127–134 (1997)
Ràfols, C., Rosés, M., Bosch, E.: Variation of acidity constants and pH values of some organic acids in water-2-propanol mixtures with solvent composition. Effect of preferential solvation. Anal. Chim. Acta 302, 109–119 (1995)
Sprunger, L.M., Aichi, S.S., Pointer, R., Acree, W.E., Jr., Abraham, M.H.: Development of Abraham model correlations for solvation characteristics of secondary and branched alcohols. Fluid Phase Equilib. 288, 121–127 (2010)
ADME Suite 5.0, Advanced Chemistry Development, 110 Yonge Street, 14th Floor, Toronto, Ontario, M5C 1T4, Canada.
Ulrich N., Endo S., Brown T. N., Watanabe, N., Bronner, G., Abraham, M. H., Goss, K-U.: UFZ-LSER database v 3.2.1 [Internet]. Leipzig, Germany, Helmholtz Centre for Environmental Research-UFZ. 2017. http://www.ufz.de/lserd
Abraham, M.H., Acree, W.E., Jr.: Solvation descriptors for zwitterionic α-aminoacids; estimation of their water–solvent partition coefficients, solubilities and hydrogen-bond acidity and hydrogen-bond basicity. ACS Omega 4, 2883–2892 (2019)
Acknowledgements
Financial support from the Spanish Ministerio de Economía y Competitividad (CTQ2017-88179-P) and the Catalan Government (2017SGR1074) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abraham, M.H., Acree, W.E., Rafols, C. et al. Equations for the Correlation and Prediction of Partition Coefficients of Neutral Molecules and Ionic Species in the Water–Isopropanol Solvent System. J Solution Chem 50, 458–472 (2021). https://doi.org/10.1007/s10953-021-01063-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01063-w