Abstract
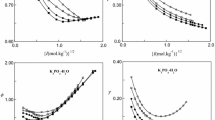
Densities of liquid phases in the water–urea–ammonium sulfamate ternary system and in the binary water–ammonium sulfamate subsystem were investigated in a wide concentration range at 288.15, 298.15 and 323.15 K. A volumetric model of the aqueous ternary solutions was proposed.






Similar content being viewed by others
References
Uchida, S., Ito, Y., Kobayashi, E.: The reaction products of ammonia and sulfur trioxide. III. The system ammonium sulfamate–ammonium sulfate–water. Nippon Kagaku Zasshi 75, 743–746 (1954)
Ricci, J.E., Selikson, B.: Aqueous solubilities of some sulfamates, and the system ammonium sulfamate–sulfarnic water at \(25\,^\circ {\rm C}\). J. Am. Chem. Soc. 69, 995–998 (1947)
Ricci, J.E., Selikson, B.: Some aqueous systems involving sulfamates. J. Am. Chem. Soc. 74, 1956–1962 (1951)
Dieter, F.F., Heinz, E.H.: Ammonium sulfate–ammonium nitrate–ammonium amidosulfate–water system. quaternary system. Wissenschaftliche Zeitschrift der Technischen Hochschule für Chemie “Carl Schorlemmer”. Leuna-Merseburg 14, 313–318 (1972)
Frederick, L.R., Starkey, R.L., Secal, W.: Decomposability of some organic sulfur compounds in soil. Soil Sci. Soc. Am. J. 21, 287–292 (1957)
Perman, E.P., Price, T.W.: Vapour pressure of concentrated aqueous solutions. Trans. Faraday Soc. 8, 68–81 (1912)
Perman, E.P., Lovett, T.: Vapour pressure and heat of dilution of aqueous solutions. Trans. Faraday Soc. 22, 1–19 (1926)
Gucker, F.T., Ayres, F.D.: The specific heats of aqueous solutions of urea from 2 to \(40^\circ \) and the apparent molal heat capacity of urea. J. Am. Chem. Soc. 59, 2152–2155 (1937)
Gucker, F.T., Gage, F.W., Moser, C.E.: Densities of aqueous solutions of urea at \(25\,^\circ {\rm C}\) and \(30\,^\circ {\rm C}\) and the apparent molal volume of urea. J. Am. Chem. Soc. 60, 2582–2588 (1938)
Wolf, A.V.: Aqueous Solutions and Body Fluids. Their Concentrative Properties and Conversion Tables. Hoeber Medical Division, Harper & Row Publishers, New York (1966)
Egan, E.P., Luff, B.B.: Heat of solution, heat capacity, and density of aqueous urea solutions at \(25\,^\circ {\rm C}\). J. Chem. Eng. Data 11, 192–194 (1966)
Boje, L., Hvidt, A.: Densities of aqueous mixtures of non-electrolytes. J. Chem. Thermodyn. 3, 663–673 (1971)
Surdo, A.L., Shin, C., Millero, F.J.: The apparent molal volume and adiabatic compressibility of some organic solutes in water at \(25\,^\circ {\rm C}\). J. Chem. Eng. Data 23, 197–201 (1978)
Singh, M., Ram, B.: Effect of urea, its concentration and temperature on water structure. Asian J. Chem. 10, 510–517 (1998)
Hakin, A.W., Beswick, C.L., Duke, M.M.: Thermochemical and volumetric properties of aqueous urea systems. Heat capacities and volumes of transfer from water to urea–water mixtures for some 1:1 electrolytes at 298.15 K. J. Chem. Soc., Faraday Trans. 92, 207–214 (1996)
Motin, M.A., Biswas, T.K., Huque, E.M.: Volumetric and viscometric studies on an aqueous urea solution. Phys. Chem. Liq. 40, 593–605 (2002)
Hagen, R., Behrends, R., Kaatze, U.: Acoustical properties of aqueous solutions of urea: Reference data for the ultrasonic spectrometry of liquids. J. Chem. Eng. Data 49, 988–991 (2004)
Singh, M., Kumar, A.: Hydrophobic interactions of methylureas in aqueous solutions estimated with density, molar volume, viscosity and surface tension from 293.15 to 303.15 K. J. Solution Chem. 35, 567–582 (2006)
Krakowiak, J., Wawer, J., Panuszko, A.: Densimetric and ultrasonic characterization of urea and its derivatives in water. J. Chem. Thermodyn. 58, 211–220 (2013)
Picker, P., Leduc, P.A., Philip, P.R., Desnoyers, J.E.: Heat capacities of solutions by flow microcalorimetry. J. Chem. Thermodyn. 3, 631–642 (1971)
Causi, S., De Lisi, R., Milioto, S., Tirone, N.: Dodecyltrimethylammonium bromide in water–urea mixtures. Volumes, heat capacities and conductivities. J. Phys. Chem. 95, 5664–5673 (1991)
Kawahara, K., Tanford, C.: Viscosity and density of aqueous solutions of urea and guanidine hydrochloride. J. Biol. Chem. 241, 3228–3232 (1966)
Brown, B.R., Gould, M.E., Ziemer, S.P., Niederhauser, T.L., Woolley, E.M.: Apparent molar volumes and apparent molar heat capacities of aqueous urea, 1,1-dimethylurea, and N,N’-dimethylurea at temperatures from (278.15 to 348.15) K and at the pressure 0.35 MPa. J. Chem. Thermodyn. 38, 1025–1035 (2006)
Schmelzle, A.F., Westfall, I.E.: The relative viscosity of aqueous solutions of sulfamic acid and of some of its salts at \(25\,^\circ {\rm C}\). J. Phys. Chem. 48, 165–168 (1944)
Synowietz, C., Schäfer, K.: Densities of Binary Aqueous Systems and Heat Capacities of Liquid Systems \({\rm N_2H_5Cl}\)–\({\rm NaJO_4}\). Landolt-Börnstein—Group IV Physical Chemistry 1b. Springer, Heidelberg (1977)
Maurey, J.R., Wolff, J.: The partial molal volumes of \({\rm OCN^-}\), \({\rm SeCN^-}\), \({\rm ReO_4^-}\) , \({\rm BF_4^-}\) , \({\rm SO_3F^-}\) , \({\rm SO_3NH_2^-}\). J. Inorg. Nucl. Chem. 25, 312–314 (1963)
Pitzer, K.S., Simonson, J.M.: Thermodynamics of multicomponent, miscible, ionic systems: theory and equations. J. Phys. Chem. 90, 3005–3009 (1986)
Clegg, S.L., Pitzer, K.S.: Thermodynamics of multicomponent, miscible, ionic solutions: generalized equations for symmetrical electrolytes. J. Phys. Chem. 96, 3513–3520 (1992)
Wagner, W., Pruß, A.: The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 31, 387–535 (2002)
Archer, D.G., Wang, P.: The dielectric constant of water and Debye-Hückel limiting law slopes. J. Phys. Chem. Ref. Data 19, 371–411 (1990)
Lide, D.R.: CRC Handbook of Chemistry and Physics, 84th edn. CRC Press LLC, Boca Raton (2003–2004)
Edward Cain, B., Kanda, F.A.: The crystal structure of ammonium sulfamate. Z. Kristallogr. 135, 253–261 (1972)
Kosova, D.A., Voskov, A.L., Kovalenko, N.A., Uspenskaya, I.A.: A water—urea—ammonium sulfamate system: Experimental investigation and thermodynamic modelling. Fluid Phase Equilibr. 425, 312–323 (2016)
Acknowledgments
The work was performed at the User Facilities Center of M.V. Lomonosov Moscow State University. The investigations were supported by the URALCHEM OJSC. The authors acknowledge partial support from the M.V. Lomonosov Moscow State University Program of Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kosova, D.A., Voskov, A.L. & Uspenskaya, I.A. Volumetric Properties of Binary and Ternary Solutions in the Water–Urea–Ammonium Sulfamate System. J Solution Chem 45, 1182–1194 (2016). https://doi.org/10.1007/s10953-016-0500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0500-z