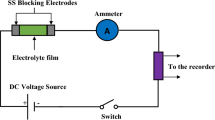
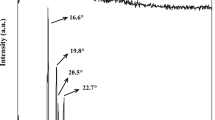
Abstract
This study investigates Li+ ion-conducting biopolymer blend electrolytes-based on chitosan (CS) and potato starch (PS) with glycerol plasticization. The advanced techniques including FTIR, impedance, TNM, LSV, and CV were employed to characterize the compositional and electrochemical properties of the solid films. The FTIR analysis indicates significant influence of glycerol on polymer/salt interactions, evidenced by the shift of FTIR bands to lower wavenumbers, signifying an increase in free ions within the host polymer system. Impedance results indicate that plasticizer addition reduces the bulk resistance to an optimum value of 49 Ω. The calculated DC values demonstrate the suitability of the electrolyte for use in energy storage applications (ESAs) with the highest ionic conductivity of 2.01 × 10−4 S cm−1. The high values of both \({\epsilon }^{{\prime }}\) and \({\epsilon }^{{\prime }{\prime }}\) at lower frequencies are due to interfacial polarization and the accumulation of charges, respectively. The sample with the largest plasticizer content has shown the highest \({\epsilon }^{{\prime }}\) of 112.4 at 105 Hz. The shifting of tan δ peaks to the higher frequency side with the increase of plasticizer indicates an increase in the mobility of cations. The combination of tan δ plot and Argand plot was used to explore the dominant mechanism in ion conduction. The electrochemical studies were performed to detect the ability of the films to be used for EDLC applications. The TNM (tion=0.947) and LSV (decomposition voltage = 3.1 V) values favor the films for ESAs. The pattern of CV curves at various scan rates established the successful design of the EDLC device. The calculated capacitance from the area under CV curves is sufficiently high. The capacitance was influenced by scan rates and changed from 12.92 to 38.68 F/g.

















Similar content being viewed by others
References
Ma X, Yu J, He K, Wang N (2007) The effects of different plasticizers on the properties of thermoplastic starch as solid polymer electrolytes. Macromol Mater Eng 292:503–510. https://doi.org/10.1002/mame.200600445
Ma X, Yu J, Zhao A (2006) Properties of biodegradable poly(propylene carbonate)/starch composites with succinic anhydride. Compos Sci Technol 66:2360–2366. https://doi.org/10.1016/j.compscitech.2005.11.028
Yoon S, Ahmed F, Zhang W, Ryu T, Jin L, Kim D et al (2020) Flexible blend polymer electrolyte membranes with excellent conductivity for fuel cells. Int J Hydrogen Energy 45:27611–27621. https://doi.org/10.1016/j.ijhydene.2020.07.076
Li L, Wang F, Li J, Yang X, You J (2017) Electrochemical performance of gel polymer electrolyte with ionic liquid and PUA/PMMA prepared by ultraviolet curing technology for lithium-ion battery. Int J Hydrogen Energy 42:12087–12093. https://doi.org/10.1016/j.ijhydene.2017.02.085
Zainuddin NK, Rasali NMJ, Mazuki NF, Saadiah MA, Samsudin AS (2020) Investigation on favourable ionic conduction based on CMC-K carrageenan proton conducting hybrid solid bio-polymer electrolytes for applications in EDLC. Int J Hydrogen Energy 45:8727–8741. https://doi.org/10.1016/j.ijhydene.2020.01.038
Singh R, Singh PK, Singh V, Bhattacharya B (2019) Quantitative analysis of ion transport mechanism in biopolymer electrolyte. Opt Laser Technol 113:303–309. https://doi.org/10.1016/j.optlastec.2018.12.036
Saadiah MA, Nagao Y, Samsudin AS (2021) Enhancement on protonation (H+) with incorporation of flexible ethylene carbonate in CMC–PVA–30 wt % NH4NO3 film. Int J Hydrogen Energy 46:17231–17245. https://doi.org/10.1016/j.ijhydene.2021.02.187
Zhou K, Zhang M, Zhang X, Wang T, Wang H, Wang Z et al (2023) A cellulose reinforced polymer composite electrolyte for the wide-temperature-range solid lithium batteries. Chem Eng J 464:142537. https://doi.org/10.1016/j.cej.2023.142537
Isa MIN, Sohaimy MIH, Ahmad NH (2021) Carboxymethyl cellulose plasticized polymer application as bio-material in solid-state hydrogen ionic cell. Int J Hydrogen Energy 46:8030–8039. https://doi.org/10.1016/j.ijhydene.2020.11.274
Nadirah BN, Ong CC, Saheed MSM, Yusof YM, Shukur MF (2020) Structural and conductivity studies of polyacrylonitrile/methylcellulose blend based electrolytes embedded with lithium iodide. Int J Hydrogen Energy 45:19590–19600. https://doi.org/10.1016/j.ijhydene.2020.05.016
Ben youcef H, Henkensmeier D, Balog S, Scherer GG, Gubler L (2020) Copolymer synergistic coupling for chemical stability and improved gas barrier properties of a polymer electrolyte membrane for fuel cell applications. Int J Hydrogen Energy 45:7059–7068. https://doi.org/10.1016/j.ijhydene.2019.12.208
Majumdar S, Sen P, Ray R (2019) Ionic interactions and transport properties in chitosan-starch based blend solid biopolymer electrolytes. Mater Today Proc 18:4913–4920. https://doi.org/10.1016/j.matpr.2019.07.483
Liang YF, Xia Y, Zhang SZ, Wang XL, Xia XH, Gu CD et al (2019) A preeminent gel blending polymer electrolyte of poly(vinylidene fluoride-hexafluoropropylene) -poly(propylene carbonate) for solid-state lithium ion batteries. Electrochim Acta 296:1064–1069. https://doi.org/10.1016/j.electacta.2018.11.182
Thayumanasundaram S, Rangasamy VS, Seo JW, Locquet JP (2017) Electrochemical performance of polymer electrolytes based on poly(vinyl alcohol)/Poly(acrylic acid) blend and pyrrolidinium ionic liquid for lithium rechargeable batteries. Electrochim Acta 240:371–378. https://doi.org/10.1016/j.electacta.2017.04.107
Abdulwahid RT, Aziz SB, Kadir MFZ (2023) Replacing synthetic polymer electrolytes in energy storage with flexible biodegradable alternatives : sustainable green biopolymer blend electrolyte for supercapacitor device. Mater Today Sustain 23:100472. https://doi.org/10.1016/j.mtsust.2023.100472
Abdulwahid RT, Aziz SB, Kadir MFZ (2023) Environmentally friendly plasticized electrolyte based on chitosan (CS): Potato starch (PS) polymers for EDLC application: steps toward the greener energy storage devices derived from biopolymers. J Energy Storage 67:107636. https://doi.org/10.1016/j.est.2023.107636
Aziz SB, Abidin ZHZ, Arof AK (2010) Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosansilver triflate electrolyte membrane. Express Polym Lett 4:300–310. https://doi.org/10.3144/expresspolymlett.2010.38
Aziz SB, Abdullah OG, Rasheed MA, Ahmed HM (2017) Effect of high salt concentration (HSC) on structural, morphological, and electrical characteristics of chitosan based solid polymer electrolytes. Polym (Basel) 9:187. https://doi.org/10.3390/polym9060187
Yassin AY (2021) Impedance, structural and thermal analyses of polyvinyl alcohol/polyvinyl pyrrolidone blend incorporated with Li + ions for lithium-ion batteries. J Mater Res Technol 15:754–767. https://doi.org/10.1016/j.jmrt.2021.08.063
Salem AM, Mohamed AR, Yassin AY (2023) The effect of low concentrations of polypyrrole on the structural, thermal, and dielectric characteristics of CMC/PPy blends. J Mater Sci Mater Electron 34:1–13. https://doi.org/10.1007/s10854-023-10938-1
Yassin AY (2023) Synthesized polymeric nanocomposites with enhanced optical and electrical properties based on gold nanoparticles for optoelectronic applications. J Mater Sci Mater Electron 34:1–18. https://doi.org/10.1007/s10854-022-09402-3
Damoom MM, Saeed A, Alshammari EM, Alhawsawi AM, Yassin AY, Abdulwahed JAM et al (2023) The role of TiO2 nanoparticles in enhancing the structural, optical, and electrical properties of PVA/PVP/CMC ternary polymer blend: nanocomposites for capacitive energy storage. J Sol-Gel Sci Technol 108:742–755. https://doi.org/10.1007/s10971-023-06223-6
Al-Muntaser AA, Pashameah RA, Saeed A, Alwafi R, Alzahrani E, AlSubhi SA et al (2023) Boosting the optical, structural, electrical, and dielectric properties of polystyrene using a hybrid GNP/Cu nanofiller: novel nanocomposites for energy storage applications. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-023-10104-7
Gupta S, Varshney PK (2017) Effect of plasticizer concentration on structural and electrical properties of hydroxyethyl cellulose (HEC)-based polymer electrolyte. Ionics (Kiel) 23:1613–1617. https://doi.org/10.1007/s11581-017-2116-8
Kumar R, Sharma S, Pathak D, Dhiman N, Arora N (2017) Ionic conductivity, FTIR and thermal studies of nano-composite plasticized proton conducting polymer electrolytes. Solid State Ionics 305:57–62. https://doi.org/10.1016/j.ssi.2017.04.020
Shin JH, Jung SS, Kim KW, Ahn HJ, Ahn JH (2002) Preparation and characterization of plasticized polymer electrolytes based on the PVdF-HFP copolymer for lithium/sulfur battery. J Mater Sci Mater Electron 13:727–733. https://doi.org/10.1023/A:1021521207247
Richardson PM, Voice AM, Ward IM (2014) Two distinct lithium diffusive species for polymer gel electrolytes containing LiBF4, propylene carbonate (PC) and PVDF. Int J Hydrogen Energy 39:2904–2908. https://doi.org/10.1016/j.ijhydene.2013.04.102
Woo HJ, Majid SR, Arof AK (2013) Effect of ethylene carbonate on proton conducting polymer electrolyte based on poly(ε-caprolactone) (PCL). Solid State Ionics 252:102–108. https://doi.org/10.1016/j.ssi.2013.07.005
Chai MN, Isa MIN (2016) Novel Proton Conducting Solid Bio-polymer Electrolytes based on Carboxymethyl Cellulose Doped with oleic acid and plasticized with glycerol. Sci Rep 6:27328. https://doi.org/10.1038/srep27328
Yusof YM, Kadir MFZ (2016) Electrochemical characterizations and the effect of glycerol in biopolymer electrolytes based on methylcellulose-potato starch blend. Mol Cryst Liq Cryst 627:220–233. https://doi.org/10.1080/15421406.2015.1137115
Shukur MF, Ithnin R, Illias HA, Kadir MFZ (2013) Proton conducting polymer electrolyte based on plasticized chitosan-PEO blend and application in electrochemical devices. Opt Mater (Amst) 35:1834–1841. https://doi.org/10.1016/j.optmat.2013.03.004
Dragunski DC, Pawlicka A (2002) Starch based solid polymeric electrolytes. Mol Cryst Liq Cryst Sci Technol Sect A Mol Cryst Liq Cryst 374:561–568. https://doi.org/10.1080/10587250210443
Bandara TMWJ, Dissanayake MAKL, Albinsson I, Mellander BE (2011) Mobile charge carrier concentration and mobility of a polymer electrolyte containing PEO and Pr4N + I- using electrical and dielectric measurements. Solid State Ionics 189:63–68. https://doi.org/10.1016/j.ssi.2011.03.004
Rice MJ, Roth WL (1972) Ionic transport in super ionic conductors: a theoretical model. J Solid State Chem 4:294–310. https://doi.org/10.1016/0022-4596(72)90121-1
Aziz SB, Karim WO, Brza MA, Abdulwahid RT, Saeed SR, Al-Zangana S et al (2019) Ion transport study in CS: POZ based polymer membrane electrolytes using Trukhan model. Int J Mol Sci. https://doi.org/10.3390/ijms20215265
Arof AK, Amirudin S, Yusof SZ, Noor IM (2014) A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys Chem Chem Phys 16:1856–1867. https://doi.org/10.1039/c3cp53830c
Abdulwahid RT, Aziz SB, Kadir MFZ (2022) Design of proton conducting solid biopolymer blend electrolytes based on chitosan-potato starch biopolymers : deep approaches to structural and ion relaxation dynamics of H + ion. J Appl Polym Sci 139:e52892. https://doi.org/10.1002/app.52892
Shukur MF, Ithnin R, Kadir MFZ (2016) Ionic conductivity and dielectric properties of potato starch-magnesium acetate biopolymer electrolytes: the effect of glycerol and 1-butyl-3-methylimidazolium chloride. Ionics (Kiel) 22:1113–1123. https://doi.org/10.1007/s11581-015-1627-4
Ibrahim S, Mohd Yasin SM, Nee NM, Ahmad R, Johan MR (2012) Conductivity and dielectric behaviour of PEO-based solid nanocomposite polymer electrolytes. Solid State Commun 152:426–434. https://doi.org/10.1016/j.ssc.2011.11.037
Liew CW, Ramesh S, Arof AK (2016) Enhanced capacitance of EDLCs (electrical double layer capacitors) based on ionic liquid-added polymer electrolytes. Energy 109:546–556. https://doi.org/10.1016/j.energy.2016.05.019
Mohit, Hashmi SA (2023) Biodegradable poly-ε-caprolactone based porous polymer electrolytes for high performance supercapacitors with carbon electrodes. J Power Sources 557:232548. https://doi.org/10.1016/j.jpowsour.2022.232548
Pistorius AMA, DeGrip WJ (2004) Deconvolution as a tool to remove fringes from an FT-IR spectrum. Vib Spectrosc 36:89–95. https://doi.org/10.1016/j.vibspec.2004.04.001
Xi J, Bai Y, Qiu X, Zhu W, Chen L, Tang X (2005) Conductivities and transport properties of microporous molecular sieves doped composite polymer electrolyte used for lithium polymer battery. New J Chem 29:1454–1460. https://doi.org/10.1039/b505332c
Abarna S, Hirankumar G (2017) Electrical, dielectric and electrochemical studies on new Li ion conducting solid polymer electrolytes based on polyethylene glycol p-tert-octylphenyl ether. Polym Sci - Ser A 59:660–668. https://doi.org/10.1134/S0965545X17050017
Ramlli MA, Bashirah NAA, Isa MIN (2018) Ionic conductivity and structural analysis of 2-hyroxyethyl cellulose doped with glycolic acid solid Biopolymer Electrolytes for Solid Proton Battery. IOP Conf Ser Mater Sci Eng 440:012038. https://doi.org/10.1088/1757-899X/440/1/012038
Brza MA, Aziz SB, Anuar H, Ali F (2020) Structural, ion transport parameter and electrochemical properties of plasticized polymer composite electrolyte based on PVA: a novel approach to fabricate high performance EDLC devices. Polym Test 91:106813. https://doi.org/10.1016/j.polymertesting.2020.106813
Aniskari NAB, Mohd Isa MIN, Bin (2017) The effect of ionic charge carriers in 2-hydroxyethyl cellulose solid biopolymer electrolytes doped glycolic acid via FTIR-deconvolution technique. J Sustain Sci Manag 2017:71–79
Mahato DK, Dutta A, Sinha TP (2010) Impedance spectroscopy analysis of double perovskite Ho 2NiTiO6. J Mater Sci 45:6757–6762. https://doi.org/10.1007/s10853-010-4771-2
Rawat P, Saroj AL (2023) Effect of ionic liquid on plasticized CS-PVP-NaI based bio-polymer blend electrolytes: structural, thermal, dielectric and ion transport properties study. Mater Sci Eng B Solid-State Mater Adv Technol 288:116215. https://doi.org/10.1016/j.mseb.2022.116215
Hamsan MH, Shukur MF, Kadir MFZ (2017) NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics (Kiel) 23:3429–3453. https://doi.org/10.1007/s11581-017-2155-1
Abdullah AM, Aziz SB, Brza MA, Saeed SR, Al-Asbahi BA, Sadiq NM et al (2022) Glycerol as an efficient plasticizer to increase the DC conductivity and improve the ion transport parameters in biopolymer based electrolytes: XRD, FTIR and EIS studies. Arab J Chem 15:103791. https://doi.org/10.1016/j.arabjc.2022.103791
Aziz SB, Woo TJ, Kadir MFZ, Ahmed HM (2018) A conceptual review on polymer electrolytes and ion transport models. J Sci Adv Mater Devices 3:1–17. https://doi.org/10.1016/j.jsamd.2018.01.002
Aziz SB, Abdulwahid RT, Kadir MFZ, Ghareeb HO, Ahamad T, Alshehri SM (2021) Design of non-faradaic EDLC from plasticized MC based Polymer electrolyte with an energy density close to lead-acid batteries. J Ind Eng Chem 105:414–426. https://doi.org/10.1016/j.jiec.2021.09.042
Abdullah AM, Aziz SB, Saeed SR (2021) Structural and electrical properties of polyvinyl alcohol (PVA):Methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab J Chem 14:103388. https://doi.org/10.1016/j.arabjc.2021.103388
Teoh KH, Lim CS, Ramesh S (2014) Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement 48:87–95. https://doi.org/10.1016/j.measurement.2013.10.040
Liew C, Ramesh S (2015) Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr Polym 124:222–228. https://doi.org/10.1016/j.carbpol.2015.02.024
Teoh KH, Lim CS, Liew CW, Ramesh S (2016) Preparation and performance analysis of barium titanate incorporated in corn starch-based polymer electrolytes for electric double layer capacitor application. J Appl Polym Sci 133:1–8. https://doi.org/10.1002/app.43275
Pal P, Ghosh A (2016) Dynamics and relaxation of charge carriers in poly(methylmethacrylate)-lithium salt based Polymer electrolytes plasticized with ethylene carbonate. J Appl Phys 120:045108. https://doi.org/10.1063/1.4959985
Shukla N, Thakur AK, Shukla A, Marx DT (2014) Ion conduction mechanism in solid polymer electrolyte: an applicability of almond-west formalism. Int J Electrochem Sci 9:7644–7659
Choudhary S, Sengwa RJ (2015) Structural and dielectric studies of amorphous and semicrystalline polymers blend-based nanocomposite electrolytes. J Appl Polym Sci 132:23–29. https://doi.org/10.1002/app.41311
Arya A, Sharma S, Sharma AL, Kumar D, Sadiq M (2016) Structural and dielectric Behaviour of Blend Polymer Electrolyte based on PEO-PAN + LiPF6. Asian J Eng Appl Technol 5:4–7. https://doi.org/10.51983/ajeat-2016.5.1.774
Bhargav PB, Mohan VM, Sharma AK, Rao VVRN (2009) Investigations on electrical properties of (PVA:NaF) Polymer electrolytes for electrochemical cell applications. Curr Appl Phys 9:165–171. https://doi.org/10.1016/j.cap.2008.01.006
Ahad N, Saion E, Gharibshahi E, Structural T (2012) Electrical properties of PVA-Sodium Salicylate Solid Composite Polymer Electrolyte. J Nanomater 2012:857569. https://doi.org/10.1155/2012/857569
Yu X, Yi B, Liu F, Wang X (2008) Prediction of the dielectric dissipation factor tan δ of polymers with an ANN model based on the DFT calculation. React Funct Polym 68:1557–1562. https://doi.org/10.1016/j.reactfunctpolym.2008.08.009
Jiang H, Hong L, Venkatasubramanian N, Grant JT, Eyink K, Wiacek K et al (2007) The relationship between chemical structure and dielectric properties of plasma-enhanced chemical vapor deposited polymer thin films. Thin Solid Films 515:3513–3520. https://doi.org/10.1016/j.tsf.2006.10.126
Baskaran R, Selvasekarapandian S, Hirankumar G, Bhuvaneswari MS (2004) Dielectric and conductivity relaxations in PVAc based Polymer electrolytes. Ionics (Kiel) 10:129–134. https://doi.org/10.1007/BF02410321
Idris NH, Senin HB, Arof AK (2007) Dielectric spectra of LiTFSI-doped chitosan/PEO blends. Ionics (Kiel) 13:213–217. https://doi.org/10.1007/s11581-007-0093-z
Smaoui H, Mir LEL, Guermazi H, Agnel S, Toureille A (2009) Study of dielectric relaxations in zinc oxide-epoxy resin nanocomposites. J Alloys Compd 477:316–321. https://doi.org/10.1016/j.jallcom.2008.10.084
Aziz SB (2016) Occurrence of electrical percolation threshold and observation of phase transition in chitosan (1 – x) :AgI x (0.05 ≤ x ≤ 0.2)-based ion-conducting solid polymer composites. Appl Phys a Mater Sci Process 122:706. https://doi.org/10.1007/s00339-016-0235-0
Aziz SB, Abidin ZHZ (2015) Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J Appl Polym Sci 132:1–10. https://doi.org/10.1002/app.41774
Belattar J, Graça MPF, Costa LC, Achour ME, Brosseau C (2010) Electric modulus-based analysis of the dielectric relaxation in carbon black loaded polymer composites. J Appl Phys. https://doi.org/10.1063/1.3452366
Pradhan DK, Choudhary RNP, Samantaray BK (2008) Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int J Electrochem Sci 3:597–608
Sengwa RJ, Choudhary S, Sankhla S (2008) Low frequency dielectric relaxation processes and ionic conductivity of montmorillonite clay nanoparticles colloidal suspension in poly(vinyl pyrrolidone)-ethylene glycol blends. Express Polym Lett 2:800–809. https://doi.org/10.3144/expresspolymlett.2008.93
Aziz SB, Abdullah OG, Rasheed MA (2017) Structural and electrical characteristics of PVA:NaTf based solid polymer electrolytes: role of lattice energy of salts on electrical DC conductivity. J Mater Sci Mater Electron 28:12873–12884. https://doi.org/10.1007/s10854-017-7117-x
Marzantowicz M, Dygas JR, Krok F, Florja??czyk Z, Zygad??o-Monikowska E (2007) Conductivity and dielectric properties of polymer electrolytes PEO:LiN(CF3SO2)2 near glass transition. J Non Cryst Solids 353:4467–4473. https://doi.org/10.1016/j.jnoncrysol.2007.04.046
Mohomed K, Gerasimov TG, Moussy F, Harmon JP (2005) A broad spectrum analysis of the dielectric properties of poly(2-hydroxyethyl methacrylate). Polym (Guildf) 46:3847–3855. https://doi.org/10.1016/j.polymer.2005.02.100
Basha SS, Rao MR (2019) Spectroscopic and Electrochemical properties of (1-x)[PVA/PVP]:x [MgCl2{6H2O}] Blend Polymer Electrolyte films. Int J Polym Sci 75:2926167
Rama Mohan K, Achari VBS, Rao VVRN, Sharma AK (2011) Electrical and optical properties of (PEMA/PVC) polymer blend electrolyte doped with NaClO4. Polym Test 30:881–886. https://doi.org/10.1016/j.polymertesting.2011.08.010
Tang J, Muchakayala R, Song S, Wang M, Kumar KN (2016) Effect of EMIMBF4 ionic liquid addition on the structure and ionic conductivity of LiBF4-complexed PVdF-HFP polymer electrolyte films. Polym Test 50:247–254. https://doi.org/10.1016/j.polymertesting.2016.01.023
Polu AR, Rhee HW, Kim DK (2015) New solid polymer electrolytes (PEO20–LiTDI–SN) for lithium batteries: structural, thermal and ionic conductivity studies. J Mater Sci Mater Electron 26:8548–8554. https://doi.org/10.1007/s10854-015-3527-9
Dhatarwal P, Choudhary S, Sengwa RJ (2018) Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries. Compos Commun 10:11–17. https://doi.org/10.1016/j.coco.2018.05.004
Rudhziah S, Rani MSA, Ahmad A, Mohamed NS, Kaddami H (2015) Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind Crops Prod 72:133–141. https://doi.org/10.1016/j.indcrop.2014.12.051
Jäckel N, Rodner M, Schreiber A, Jeongwook J, Zeiger M, Aslan M et al (2016) Anomalous or regular capacitance? The influence of pore size dispersity on double-layer formation. J Power Sources 326:660–671. https://doi.org/10.1016/j.jpowsour.2016.03.015
He X, Lei J, Geng Y, Zhang X, Wu M, Zheng M (2009) Preparation of microporous activated carbon and its electrochemical performance for electric double layer capacitor. J Phys Chem Solids 70:738–744. https://doi.org/10.1016/j.jpcs.2009.03.001
Fang B, Binder L (2006) A novel carbon electrode material for highly improved EDLC performance. J Phys Chem B 110:7877–7882. https://doi.org/10.1021/jp060110d
Hamsan MH, Shukur MF, Aziz SB, Yusof YM, Kadir MFZ (2020) Influence of NH4Br as an ionic source on the structural/electrical properties of dextran-based biopolymer electrolytes and EDLC application. Bull Mater Sci. https://doi.org/10.1007/s12034-019-2008-9
Teoh KH, Lim CS, Liew CW, Ramesh S, Ramesh S (2015) Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics (Kiel) 21:2061–2068. https://doi.org/10.1007/s11581-014-1359-x
Asnawi ASFM, Hamsan MH, Kadir FZ, Aziz SB, Matmin J (2021) Impregnation of [Emim]Br ionic liquid as plasticizer in biopolymer electrolytes for EDLC application. Electrochim Acta 375:137923. https://doi.org/10.1016/j.electacta.2021.137923
Aziz SB, Hamsan MH, Brza MA, Kadir MFZ, Muzakir SK, Abdulwahid RT (2020) Effect of glycerol on EDLC characteristics of chitosan:methylcellulose polymer blend electrolytes. J Mater Res Technol 9:8355–8366. https://doi.org/10.1016/j.jmrt.2020.05.114
Lim CS, Teoh KH, Liew CW, Ramesh S (2014) Capacitive behavior studies on electrical double layer capacitor using poly (vinyl alcohol)-lithium perchlorate based Polymer electrolyte incorporated with TiO2. Mater Chem Phys 143:661–667. https://doi.org/10.1016/j.matchemphys.2013.09.051
Liew CW, Ramesh S, Arof AK (2015) Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int J Hydrogen Energy 40:852–862. https://doi.org/10.1016/j.ijhydene.2014.09.160
Kadir MFZ, Arof AK (2013) Application of PVA-chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater Res Innov 15:S217–S220. https://doi.org/10.1179/143307511X13031890749299
Bashir S, Omar FS, Hina M, Numan A, Iqbal J, Ramesh S et al (2020) Synthesis and characterization of hybrid poly (N, N-dimethylacrylamide) composite hydrogel electrolytes and their performance in supercapacitor. Electrochim Acta 332:135438. https://doi.org/10.1016/j.electacta.2019.135438
Acknowledgements
The authors gratefully acknowledge all the support for this study from the Ministry of Higher Education and Scientific Research-Kurdish National Research Council (KNRC), Kurdistan Regional Government. The support from the University of Sulaimani, University of Humburg, University of Charmo, University of Cihan Sulaimaniya, University of Raparin and University of Malaya are greatly acknowledged. The authors express their gratitude to the Researchers Supporting Project Number (RSP2024R348), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, SB; Data curation, RT; Formal analysis, DM, SB and RT; Funding acquisition, BA; Investigation, RT, SA and PS; Methodology, SA, HJ, RT and AA; Project administration, SB and MF; Resources, BA and HJ ; Supervision, SB and MK; Validation, DM, SA, PS, WO, BA, AA and HJ ; Visualization, DM and RT; Writing—original draft, DM and SB; Writing—review & editing, RT, SA, WO, PS, HJ, and A
Corresponding authors
Ethics declarations
Conflict of interest
The authors assert that they have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aziz, D.M., Abdulwahid, R.T., Hassan, S.A. et al. Development and Investigation of Electrochemical and Dielectric Properties of Eco-Friendly Lithium-Ion Conductor Biopolymer Electrolyte for Energy Storage Application. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03198-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03198-5