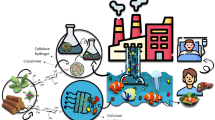
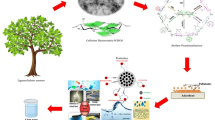
Abstract
This study aimed to investigate the adsorption of As(V), phosphate, and textile dye Acid Green 25 (AG-25) on layered double hydroxides Mn-Fe_LDH and corresponding membranes (wCell/Mn-Fe_LDH). The wCell membrane, derived from waste tobacco boxes, was formed by cross-linking of epoxy and amino modified cellulose fibers with epoxy modified Mn-Fe_LDH and lysine as cross-linker. Structural and morphological analyses were conducted for Mn-Fe_LDH and wCell/Mn-Fe_LDH. The batch system explored pH, contact time, temperature, and initial concentration effects on wCell/Mn-Fe_LDH adsorption efficiency. Adsorption capacities of 82.71, 106.9, and 130.3 mg g−1 were achieved for As(V), phosphate, and AG-25, respectively, indicating effective anionic species removal. Kinetic analysis suggested intraparticle diffusion as the rate-limiting step. Thermodynamic parameters and ionic strength effects indicated a physisorption mechanism for AG-25 and surface complexation for As(V) and phosphate. Biodegradation experiments after five adsorption/desorption cycles revealed the membrane’s decomposition, with phosphate’s strong bonding releasing essential elements valuable for soil fertilization. Effluent wastewater treatment demonstrated low environmental impact through the formation of insoluble As(V) salts and photocatalytic dye degradation.









Similar content being viewed by others
References
Bukhtiyarova MV (2019) A review on effect of synthesis conditions on the formation of layered double hydroxides. J Solid State Chem 269:494–506. https://doi.org/10.1016/j.jssc.2018.10.018
Hibino T (2018) Anion selectivity of layered double hydroxides: effects of crystallinity and charge density. Eur J Inorg Chem 2018:722–730. https://doi.org/10.1002/ejic.201701067
Sun X, Neuperger E, Dey SK (2015) Insights into the synthesis of layered double hydroxide (LDH) nanoparticles: part 1. Optimization and controlled synthesis of chloride-intercalated LDH. J Colloid Interface Sci 459:264–272. https://doi.org/10.1016/j.jcis.2015.07.073
Constantino VRL, Pinnavaia TJ (1995) Basic properties of Mg2 + 1-xAl3 + x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg Chem 34:883–892. https://doi.org/10.1021/ic00108a020
Sajid M, Basheer C (2016) Layered double hydroxides: emerging sorbent materials for analytical extractions. TrAC Trends Anal Chem 75:174–182. https://doi.org/10.1016/j.trac.2015.06.010
Meng Z, Zhang Y, Zhang Q et al (2017) Novel synthesis of layered double hydroxides (LDHs) from zinc hydroxide. Appl Surf Sci 396:799–803. https://doi.org/10.1016/j.apsusc.2016.11.032
Zümreoglu-Karan B, Ay A (2012) Layered double hydroxides — multifunctional nanomaterials. Chem Pap 66:1–10. https://doi.org/10.2478/s11696-011-0100-8
Othman MR, Helwani Z, Martunus, Fernando WJN (2009) Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: a review. Appl Organomet Chem 23:335–346. https://doi.org/10.1002/aoc.1517
Yang H, Kang J-K, Jeong S et al (2022) Removal of perfluorooctanoic acid from water using peroxydisulfate/layered double hydroxide system: optimization using response surface methodology and artificial neural network. Process Saf Environ Prot 167:368–377. https://doi.org/10.1016/j.psep.2022.09.032
Theiss FL, Ayoko GA, Frost RL (2016) Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2 + and Al3 + layer cations by co-precipitation methods—A review. Appl Surf Sci 383:200–213. https://doi.org/10.1016/j.apsusc.2016.04.150
Alloway BJ (2013) Heavy metals in Soils. Springer Netherlands, Dordrecht
Millaleo R, Reyes- Diaz M, Ivanov A et al (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J soil Sci Plant Nutr 10:470–481. https://doi.org/10.4067/S0718-95162010000200008
Aleksandra D, Papludis SČA (2018) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 59:385–393. https://doi.org/10.5937/ZasMat1803385
Manzar MS, Aziz HA, Meili L et al (2023) Insights into the adsorption of tetracycline onto cellulose nanocrystal structured MgAl/LDH composite. Mater Chem Phys 299:127247. https://doi.org/10.1016/j.matchemphys.2022.127247
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173. https://doi.org/10.1016/j.watres.2016.01.008
Yan CY, Yi WT (2013) Boron adsorption by cellulose supported layered double hydroxides. Adv Mater Res 807–809:1380–1383. https://doi.org/10.4028/www.scientific.net/AMR.807-809.1380
d’Halluin M, Rull-Barrull J, Bretel G et al (2017) Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustain Chem Eng 5:1965–1973. https://doi.org/10.1021/acssuschemeng.6b02768
Bessaies H, Iftekhar S, Asif MB et al (2021) Characterization and physicochemical aspects of novel cellulose-based layered double hydroxide nanocomposite for removal of antimony and fluoride from aqueous solution. J Environ Sci (China) 102:301–315. https://doi.org/10.1016/j.jes.2020.09.034
Bessaies H, Iftekhar S, Doshi B et al (2020) Synthesis of novel adsorbent by intercalation of biopolymer in LDH for the removal of arsenic from synthetic and natural water. J Environ Sci (China) 91:246–261. https://doi.org/10.1016/j.jes.2020.01.028
Yue X, Li J, Zhang T et al (2017) In situ one-step fabrication of durable superhydrophobic-superoleophilic cellulose/LDH membrane with hierarchical structure for efficiency oil/water separation. Chem Eng J 328:117–123. https://doi.org/10.1016/j.cej.2017.07.026
Jiang Z, Luo P, Xie C, Zhang A (2021) Facile construction of cellulose/layered double hydroxides nanocomposite membranes with high strength and antibacterial properties. J Appl Polym Sci 138:51845. https://doi.org/10.1002/app.51845
Villarín MC, Merel S (2020) Paradigm shifts and current challenges in wastewater management. J Hazard Mater 390:122139. https://doi.org/10.1016/j.jhazmat.2020.122139
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F et al (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504. https://doi.org/10.1016/j.eti.2021.101504
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Almanassra IW, Mckay G, Kochkodan V et al (2021) A state of the art review on phosphate removal from water by biochars. Chem Eng J 409:128211. https://doi.org/10.1016/j.cej.2020.128211
Ji X, Ye C, Zhou J et al (2021) Study on the microscale structure and barrier mechanism of magnesium phosphate cement modified with fly ash cutoff walls for lead pollution in groundwater. Constr Build Mater 308:124994. https://doi.org/10.1016/j.conbuildmat.2021.124994
Tajat N, El Hayaoui W, El Mouhri W et al (2023) Simultaneous removal of anionic and cationic dyes from aqueous solutions using nickel–iron layered double hydroxide nanosheets. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-023-05155-6
Moon S, Ryu J, Hwang J, Lee C-G (2023) Efficient removal of dyes from aqueous solutions using short-length bimodal mesoporous carbon adsorbents. Chemosphere 313:137448. https://doi.org/10.1016/j.chemosphere.2022.137448
Faisal AAH, Shihab AH, Naushad M et al (2021) Green synthesis for novel sorbent of sand coated with (Ca/Al)-layered double hydroxide for the removal of toxic dye from aqueous environment. J Environ Chem Eng 9:105342. https://doi.org/10.1016/j.jece.2021.105342
Zubair M, Ihsanullah I, Abdul Aziz H et al (2021) Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour Technol 319:124128. https://doi.org/10.1016/j.biortech.2020.124128
Ahmed DN, Naji LA, Faisal AAH et al (2020) Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci Rep 10:2042. https://doi.org/10.1038/s41598-020-58866-y
Abbasi M, Sabzehmeidani MM, Ghaedi M et al (2021) Synthesis of grass-like structured Mn-Fe layered double hydroxides/PES composite adsorptive membrane for removal of malachite green. Appl Clay Sci 203:105946. https://doi.org/10.1016/j.clay.2020.105946
Shi Z, Wang Y, Sun S et al (2020) Removal of methylene blue from aqueous solution using Mg-Fe, Zn-Fe, Mn-Fe layered double hydroxide. Water Sci Technol 81:2522–2532. https://doi.org/10.2166/wst.2020.313
Wang Y, Gao Y, Zhu Z et al (2021) Enhanced Arsenic removal from Aqueous Solution by Fe/Mn-C layered double Hydroxide Composite. Adsorpt Sci Technol 2021:1–12. https://doi.org/10.1155/2021/8891643
Tian Y, Liu G, Gao Y et al (2021) Comparative study on as(III) and as(V) adsorption by CO23–intercalated Fe/Mn-LDHs from aqueous solution. Blue-Green Syst 3:175–190. https://doi.org/10.2166/bgs.2021.010
Otgonjargal E, Kim YS, Park SM et al (2012) Mn-Fe layered double hydroxides for Adsorption of as(III) and as(V). Sep Sci Technol 47:2192–2198. https://doi.org/10.1080/01496395.2012.697509
Liu G, Zhu Z, Zhao N et al (2020) Mn-Fe layered double Hydroxide Intercalated with Ethylene-Diaminetetraacetate Anion: synthesis and removal of as(III) from aqueous solution around pH 2–11. Int J Environ Res Public Health 17:9341. https://doi.org/10.3390/ijerph17249341
Perendija J, Veličković ZS, Cvijetić I et al (2021) Bio-membrane based on modified cellulose, lignin, and tannic acid for cation and oxyanion removal: experimental and theoretical study. Process Saf Environ Prot 147:609–625. https://doi.org/10.1016/j.psep.2020.12.027
Perendija J, Veličković ZS, Cvijetić I et al (2020) Batch and column adsorption of cations, oxyanions and dyes on a magnetite modified cellulose-based membrane. Cellulose 27:8215–8235. https://doi.org/10.1007/s10570-020-03352-x
Fritsch S, Sarrias J, Rousset A, Kulkarni G (1998) Low-temperature oxidation of Mn3O4 hausmannite. Mater Res Bull 33:1185–1194. https://doi.org/10.1016/S0025-5408(98)00108-1
Hem JD (1981) Rates of manganese oxidation in aqueous systems. Geochim Cosmochim Acta 45:1369–1374. https://doi.org/10.1016/0016-7037(81)90229-5
Davies SH, Morgan JJ (1989) Manganese(II) oxidation kinetics on metal oxide surfaces. J Colloid Interface Sci 129:63–77. https://doi.org/10.1016/0021-9797(89)90416-5
Nguyen TH, Tran HN, Nguyen TV et al (2022) Single-step removal of arsenite ions from water through oxidation-coupled adsorption using Mn/Mg/Fe layered double hydroxide as catalyst and adsorbent. Chemosphere 295:133370. https://doi.org/10.1016/j.chemosphere.2021.133370
Biesinger MC, Lau LWM, Gerson AR, Smart RSC (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: sc, Ti, V, Cu and Zn. Appl Surf Sci 257:887–898. https://doi.org/10.1016/j.apsusc.2010.07.086
Mullet M, Khare V, Ruby C (2008) XPS study of Fe(II)Fe(III) (oxy)hydroxycarbonate green rust compounds. Surf Interface Anal 40:323–328. https://doi.org/10.1002/sia.2758
Biesinger MC, Payne BP, Grosvenor AP et al (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257:2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Kloprogge JT, Wood BJ (2020) Handbook of mineral spectroscopy. Elsevier, Amsterdam. https://doi.org/10.1016/C2015-0-01704-X
Ilton ES, Post JE, Heaney PJ et al (2016) XPS determination of mn oxidation states in Mn (hydr)oxides. Appl Surf Sci 366:475–485. https://doi.org/10.1016/j.apsusc.2015.12.159
Xie G, Liu X, Li Q et al (2017) The evolution of α-MnO2 from hollow cubes to hollow spheres and their electrochemical performance for supercapacitors. J Mater Sci 52:10915–10926. https://doi.org/10.1007/s10853-017-1116-4
ADMET Predictor (2016) Simulations Plus, Inc, Lancaster, CA, USA, ver. 8
Peter Guthr J (1978) Hydrolysis of esters of oxy acids: pKa values for strong acids; brflnsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a ra. E Can J Chem 56:2342
Vuković GD, Marinković AD, Škapin SD et al (2011) Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem Eng J 173:855–865. https://doi.org/10.1016/j.cej.2011.08.036
Ta HTT, Tieu AK, Zhu H et al (2018) Chemical origin of sodium phosphate interactions on iron and iron oxide surfaces by first principle calculations. J Phys Chem C 122:635–647. https://doi.org/10.1021/acs.jpcc.7b10731
Veličković Z, Vuković GD, Marinković AD et al (2012) Adsorption of arsenate on iron(III) oxide coated ethylenediamine functionalized multiwall carbon nanotubes. Chem Eng J 181–182:174–181. https://doi.org/10.1016/j.cej.2011.11.052
Taleb K, Markovski J, Veličković Z et al (2019) Arsenic removal by magnetite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab J Chem 12:4675–4693. https://doi.org/10.1016/j.arabjc.2016.08.006
Markovski JS, Marković DD, Đokić VR et al (2014) Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem Eng J 237:430–442. https://doi.org/10.1016/j.cej.2013.10.031
Kaygusuz H, Uzaşçı S, Erim FB (2015) Removal of Fluoride from Aqueous Solution using aluminum alginate beads. CLEAN - Soil Air Water 43:724–730. https://doi.org/10.1002/clen.201300632
Inglezakis VJ, Zorpas AA (2012) Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin Water Treat 39:149–157. https://doi.org/10.1080/19443994.2012.669169
Sairam Sundaram C, Viswanathan N, Meenakshi S (2008) Uptake of fluoride by nano-hydroxyapatite/chitosan, a bioinorganic composite. Bioresour Technol 99:8226–8230. https://doi.org/10.1016/j.biortech.2008.03.012
Sundaram CS, Viswanathan N, Meenakshi S (2008) Defluoridation chemistry of synthetic hydroxyapatite at nano scale: equilibrium and kinetic studies. J Hazard Mater 155:206–215. https://doi.org/10.1016/j.jhazmat.2007.11.048
Lee C-G, Kim J-H, Kang J-K et al (2015) Comparative analysis of fixed-bed sorption models using phosphate breakthrough curves in slag filter media. Desalin Water Treat 55:1795–1805. https://doi.org/10.1080/19443994.2014.930698
Yakout SME, Abdeltawab AA, Elhindi K, Askalany A (2018) Uranium dynamic adsorption breakthrough curve onto Rice Straw based activated Carbon using Bed depth Service Time Model. BioResources 13:9143–9157. https://doi.org/10.15376/BIORES.13.4.9143-9157
Feng L, Zhang Q, Ji F et al (2022) Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol / lanthanum alginate hydrogels. Chem Eng J 430:132754. https://doi.org/10.1016/j.cej.2021.132754
Taleb K, Markovski J, Milosavljević M et al (2015) Efficient arsenic removal by cross-linked macroporous polymer impregnated with hydrous iron oxide: material performance. Chem Eng J 279:66–78. https://doi.org/10.1016/j.cej.2015.04.147
Karanac M, Đolić M, Veljović Đ et al (2018) The removal of Zn2+, Pb2+, and as(V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag 78:366–378. https://doi.org/10.1016/j.wasman.2018.05.052
Nikolić V, Tomić N, Bugarčić M et al (2021) Amino-modified hollow alumina spheres: effective adsorbent for Cd2+, Pb2+, as(V), and diclofenac removal. Environ Sci Pollut Res 28:27174–27192. https://doi.org/10.1007/s11356-020-12157-1
Akpan UG, Hameed BH (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529. https://doi.org/10.1016/j.jhazmat.2009.05.039
Frimmel FH GA-B (2011) Treatise on Water Science. Academic Press Professional, Oxford
Park CH, Kang YK, Im SS (2004) Biodegradability of cellulose fabrics. J Appl Polym Sci 94:248–253. https://doi.org/10.1002/app.20879
Hokkanen S, Repo E, Lou S, Sillanpää M (2015) Removal of arsenic(V) by magnetic nanoparticle activated microfibrillated cellulose. Chem Eng J 260:886–894. https://doi.org/10.1016/j.cej.2014.08.093
Seftel EM, Ciocarlan RG, Michielsen B et al (2018) Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides. Appl Clay Sci 165:234–246. https://doi.org/10.1016/j.clay.2018.08.018
Khitous M, Salem Z, Halliche D (2016) Removal of phosphate from industrial wastewater using uncalcined MgAl-NO 3 layered double hydroxide: batch study and modeling. Desalin Water Treat 57:15920–15931. https://doi.org/10.1080/19443994.2015.1077745
Acknowledgements
This work was supported by the Ministry of Science, Technological Development and Innovations of the Republic of Serbia (Contract Grants No. 451-03-47/2023-01/200135, 451-03-47/2023-01/200017, and 451-03-47/2023-01/200326).
Author information
Authors and Affiliations
Contributions
M. A. A.: Conceptualization, Investigation, Data curation, Writing—original draft preparation; M. M. V.: Investigation, Formal analysis, Writing—reviewing and editing; M. M.: Investigation, Visualization, Formal analysis, Writing—reviewing and editing; A. E.: Investigation, Formal analysis; A. S.: Investigation Validation, Data Curation; Z. V.: Investigation, Data Curation; A. M.: Conceptualization, Resources, Validation, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abduarahman, M.A., Vuksanović, M.M., Milošević, M. et al. Mn-Fe Layered Double Hydroxide Modified Cellulose-Based Membrane for Sustainable Anionic Pollutant Removal. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03192-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03192-x