Abstract
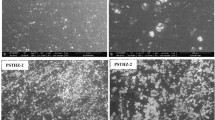
In this study, we report polyvinyl alcohol/carboxymethyl cellulose/gelatin/ZSM-5 zeolite (PVA/CMC/GEL/ZSM-5) membrane for cationic dye (rhodamine B, Rh B) removal from aqueous solution. The prepared membrane was characterized using different techniques such as Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), optical microscopy (OM), universal testing machine (UTM) and water contact angle respectively. XRD, FT-IR and SEM analysis indicates successful incorporation of zeolite into PVA/CMC/GEL membrane. The improved hydrophobicity of the zeolite loaded membrane was confirmed by contact angle analysis. The Rh B removal efficiency of zeolite loaded PVA/CMC/GEL membrane was investigated through batch adsorption technique. The effect of different parameters such as initial dye concentration, zeolite dosage, contact time, temperature and pH on the adsorption was examined. Rh B dye adsorption onto the membrane followed Freundlich isotherm model. The kinetic studies revealed that Rh B dye adsorption on the membrane could be explained using pseudo-second-order model. Finally, the recyclability test revealed that the membrane exhibits good recycle efficiency and is stable after six recycle.











Similar content being viewed by others
References
Gleick PH (1998) Water in crisis: paths to sustainable water use. Ecol Appl 8:571–579
Asadi S, Eris S, Azizian S (2018) Alginate-based hydrogel beads as a biocompatible and efficient adsorbent for dye removal from aqueous solutions. ACS Omega 3:15140–15148
Mohamed RR, Abu Elella MH, Sabaa MW, Saad GR (2018) Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 25:6513–6529
Talaiekhozani A, Rezania S (2017) Application of photosynthetic bacteria for removal of heavy metals, macro-pollutants and dye from wastewater: a review. J Water Process Eng 19:312–321
Kahlon SK, Sharma G, Julka JM, Kumar A, Sharma S, Stadler FJ (2018) Impact of heavy metals and nanoparticles on aquatic biota. Environ Chem Lett 16:919–946
Duman O, Polat TG, Diker CÖ, Tunç S (2020) Agar/κ-carrageenan composite hydrogel adsorbent for the removal of methylene blue from water. Int J Biol Macromol 160:823–835
Ali H (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213:251–273
Piaskowski K, Świderska-Dąbrowska R, Zarzycki PK (2018) Dye removal from water and wastewater using various physical, chemical, and biological processes. J AOAC Int 101:1371–1384
Gürses A, Açıkyıldız M, Güneş K, Gürses MS (2016) Dyes and pigments: their structure and properties. Dyes and pigments. Springer, Cham, pp 13–29
Shabbir M (2019) Textiles and clothing. Wiley, Hoboken
He K, Chen G, Zeng G, Chen A, Huang Z, Shi J, Peng M, Huang T, Hu L (2018) Enhanced removal performance for methylene blue by kaolin with graphene oxide modification. J Taiwan Inst Chem Eng 89:77–85
Khan R, Bhawana P, Fulekar MH (2012) Microbial decolorization and degradation of synthetic dyes: a review. Rev Environ Sci Bio/Technol 12:75–97
Sabarish R, Unnikrishnan G (2018) Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr Polym 199:129–140
Radoor S, Karayil J, Jayakumar A, Parameswaranpillai J, Siengchin S (2021) An efficient removal of malachite green dye from aqueous environment using ZSM-5 zeolite/polyvinyl alcohol/carboxymethyl cellulose/sodium alginate bio composite. J Polym Environ 29:2126–2139
Radoor S, Karayil J, Parameswaranpillai J, Siengchin S (2020) Adsorption study of anionic dye, eriochrome black T from aqueous medium using polyvinyl alcohol/starch/ZSM-5 zeolite membrane. J Polym Environ 28:2631–2643
Zhu M-X, Lee L, Wang H-H, Wang Z (2007) Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J Hazard Mater 149:735–741
Yi H, Huang D, Qin L, Zeng G, Lai C, Cheng M, Ye S, Song B, Ren X, Guo X (2018) Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl Catal B 239:408–424
Xu D, Cheng B, Cao S, Yu J (2015) Enhanced photocatalytic activity and stability of Z-scheme Ag2CrO4-GO composite photocatalysts for organic pollutant degradation. Appl Catal B 164:380–388
Radoor S, Karayil J, Parameswaranpillai J, Siengchin S (2020) Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci Rep. https://doi.org/10.1038/s41598-020-72398-5
Sabarish R, Jasila K, Aswathy J, Jyotishkumar P, Suchart S (2020) Fabrication of PVA/agar/modified ZSM-5 zeolite membrane for removal of anionic dye from aqueous solution. Int J Environ Sci Technol 18:2571–2586
Anush SM, Chandan HR, Vishalakshi B (2019) Synthesis and metal ion adsorption characteristics of graphene oxide incorporated chitosan Schiff base. Int J Biol Macromol 126:908–916
Moosavi S, Lai CW, Gan S, Zamiri G, Akbarzadeh Pivehzhani O, Johan MR (2020) Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 5:20684–20697
Radoor S, Karayil J, Parameswaranpillai J, Siengchin S (2020) Adsorption of methylene blue dye from aqueous solution by a novel PVA/CMC/halloysite nanoclay bio composite: characterization, kinetics, isotherm and antibacterial properties. J Environ Health Sci Eng 18:1311–1327
Huang T, Yan M, He K, Huang Z, Zeng G, Chen A, Peng M, Li H, Yuan L, Chen G (2019) Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J Colloid Interface Sci 543:43–51
Anush SM, Chandan HR, Gayathri BH, Asma, Manju N, Vishalakshi B, Kalluraya B (2020) Graphene oxide functionalized chitosan-magnetite nanocomposite for removal of Cu(II) and Cr(VI) from waste water. Int J Biol Macromol 164:4391–4402
Radoor S, Karayil J, Jayakumar A, Parameswaranpillai J, Siengchin S (2021) Removal of methylene blue dye from aqueous solution using PDADMAC modified ZSM-5 zeolite as a novel adsorbent. J Polym Environ 29:3185–3198
Sabarish R, Unnikrishnan G (2020) A novel anionic surfactant as template for the development of hierarchical ZSM-5 zeolite and its catalytic performance. J Porous Mater 27:691–700
Radoor S, Karayil J, Jayakumar A, Parameswaranpillai J, Siengchin S (2021) Efficient removal of methyl orange from aqueous solution using mesoporous ZSM-5 zeolite: Synthesis, kinetics and isotherm studies. Colloids Surf A 611:125852
Sabarish R, Unnikrishnan G (2019) Synthesis, characterization and evaluations of micro/mesoporous ZSM-5 zeolite using starch as bio template. SN Appl Sci. https://doi.org/10.1007/s42452-019-1036-9
Sabarish R, Unnikrishnan G (2018) Novel biopolymer templated hierarchical silicalite-1 as an adsorbent for the removal of rhodamine B. J Mol Liq 272:919–929
Sabarish R, Unnikrishnan G (2017) Synthesis, characterization and catalytic activity of hierarchical ZSM-5 templated by carboxymethyl cellulose. Powder Technol 320:412–419
Wojciechowska KM, Król M, Bajda T, Mozgawa W (2019) Sorption of heavy metal cations on mesoporous ZSM-5 and mordenite zeolites. Materials. https://doi.org/10.3390/ma12193271
Sabarish R, Unnikrishnan G (2018) PVA/PDADMAC/ZSM-5 zeolite hybrid matrix membranes for dye adsorption: fabrication, characterization, adsorption, kinetics and antimicrobial properties, Journal of Environmental. Chem Eng 6:3860–3873
Khamparia S, Jaspal DK (2017) Xanthium strumarium L. seed hull as a zero cost alternative for Rhodamine B dye removal. J Environ Manage 197:498–506
Das SK, Bhowal J, Das AR, Guha AK (2006) Adsorption behavior of rhodamine B on rhizopus oryzae biomass. Langmuir 22:7265–7272
Ramesha GK, Vijaya Kumara A, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361:270–277
Shakir K, Elkafrawy AF, Ghoneimy HF, Elrab Beheir SG, Refaat M (2010) Removal of rhodamine B (a basic dye) and thoron (an acidic dye) from dilute aqueous solutions and wastewater simulants by ion flotation. Water Res 44:1449–1461
Khamparia S, Jaspal D (2016) Investigation of adsorption of rhodamine B onto a natural adsorbent Argemone mexicana. J Environ Manage 183:786–793
Kooh MRR, Lim LBL, Lim LH, Dahri MK (2016) Separation of toxic rhodamine B from aqueous solution using an efficient low-cost material, Azolla pinnata, by adsorption method. Environ Monit Assess. https://doi.org/10.1007/s10661-016-5108-7
Rasalingam S, Peng R, Koodali RT (2013) An investigation into the effect of porosities on the adsorption of rhodamine B using titania–silica mixed oxide xerogels. J Environ Manage 128:530–539
Soltani T, Entezari MH (2013) Sono-synthesis of bismuth ferrite nanoparticles with high photocatalytic activity in degradation of rhodamine B under solar light irradiation. Chem Eng J 223:145–154
Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL (2014) Activated carbon developed from orange peels: batch and dynamic competitive adsorption of basic dyes. Ind Crops Prod 62:437–445
da Rosa ALD, Carissimi E, Dotto GL, Sander H, Feris LA (2018) Biosorption of rhodamine B dye from dyeing stones effluents using the green microalgae Chlorella pyrenoidosa. J Clean Prod 198:1302–1310
Santhi T, Prasad AL, Manonmani S (2014) A comparative study of microwave and chemically treated Acacia nilotica leaf as an eco friendly adsorbent for the removal of rhodamine B dye from aqueous solution. Arab J Chem 7:494–503
Chieng HI, Lim LBL, Priyantha N (2014) Sorption characteristics of peat from Brunei Darussalam for the removal of rhodamine B dye from aqueous solution: adsorption isotherms, thermodynamics, kinetics and regeneration studies. Desalin Water Treat 55:664–677
Habiba U, Siddique TA, Joo TC, Salleh A, Ang BC, Afifi AM (2017) Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr Polym 157:1568–1576
Habiba U, Siddique TA, Li Lee JJ, Joo TC, Ang BC, Afifi AM (2018) Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr Polym 191:79–85
Baheri B, Ghahremani R, Peydayesh M, Shahverdi M, Mohammadi T (2015) Dye removal using 4A-zeolite/polyvinyl alcohol mixed matrix membrane adsorbents: preparation, characterization, adsorption, kinetics, and thermodynamics. Res Chem Intermed 42:5309–5328
Shamsudin MS, Azha SF, Shahadat M, Ismail S (2019) Cellulose/bentonite-zeolite composite adsorbent material coating for treatment of N-based antiseptic cationic dye from water. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2019.02.004
Prasad CV, Swamy BY, Sudhakar H, Sobharani T, Sudhakar K, Subha MCS, Rao KC (2011) Preparation and characterization of 4A zeolite-filled mixed matrix membranes for pervaporation dehydration of isopropyl alcohol. J Appl Polym Sci 121:1521–1529
Prasad CV, Yeriswamy B, Sudhakar H, Sudhakara P, Subha MCS, Song JI, Rao KC (2012) Preparation and characterization of nanoparticle-filled, mixed-matrix membranes for the pervaporation dehydration of isopropyl alcohol. J Appl Polym Sci 125:3351–3360
Narayanan S, Vijaya JJ, Sivasanker S, Ragupathi C, Sankaranarayanan TM, Kennedy LJ (2016) Hierarchical ZSM-5 catalytic performance evaluated in the selective oxidation of styrene to benzaldehyde using TBHP. J Porous Mater 23:741–752
Bai P, Wu P, Xing W, Liu D, Zhao L, Wang Y, Xu B, Yan Z, Zhao XS (2015) Synthesis and catalytic properties of ZSM-5 zeolite with hierarchical pores prepared in the presence of n-hexyltrimethylammonium bromide. J Mater Chem A 3:18586–18597
Teli SB, Calle M, Li N (2011) Poly(vinyl alcohol)-H-ZSM-5 zeolite mixed matrix membranes for pervaporation separation of methanol–benzene mixture. J Membr Sci 371:171–178
Amirilargani M, Sadatnia B (2014) Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J Membr Sci 469:1–10
Fan X, Yu L, Li L, Yang C, Wen J, Ye X, Cheng J, Hu Y (2017) Characterization and application of zeolitic imidazolate framework-8@polyvinyl alcohol nanofibers mats prepared by electrospinning. Mater Res Express 4:026404
Veerapur RS, Patil MB, Gudasi KB, Aminabhavi TM (2008) Poly(vinyl alcohol)–zeolite T mixed matrix composite membranes for pervaporation separation of water+1,4-dioxane mixtures. Sep Purif Technol 58:377–385
Alver E, Metin AÜ (2012) Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies. Chem Eng J 200–202:59–67
Liu S, Ding Y, Li P, Diao K, Tan X, Lei F, Zhan Y, Li Q, Huang B, Huang Z (2014) Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N, N-dimethyl dehydroabietylamine oxide. Chem Eng J 248:135–144
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148:354–364
Liu Y, Zheng Y, Wang A (2010) Enhanced adsorption of methylene blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J Environ Sci 22:486–493
Han R, Zhang J, Han P, Wang Y, Zhao Z, Tang M (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Sohrabnezhad S, Pourahmad A (2010) Comparison absorption of new methylene blue dye in zeolite and nanocrystal zeolite. Desalination 256:84–89
Fil BA, Ozmetin C, Korkmaz M (2012) Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull Korean Chem Soc 33:3184–3190
Zhang L, Zhang H, Guo W, Tian Y (2014) Removal of malachite green and crystal violet cationic dyes from aqueous solution using activated sintering process red mud. Appl Clay Sci 93–94:85–93
Foroughi-Dahr M, Abolghasemi H, Esmaili M, Shojamoradi A, Fatoorehchi H (2014) Adsorption characteristics of congo red from aqueous solution onto tea waste. Chem Eng Commun 202:181–193
Mane VS, Vijay Babu PV (2013) Kinetic and equilibrium studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust. J Taiwan Inst Chem Eng 44:81–88
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119
Hor KY, Chee JMC, Chong MN, Jin B, Saint C, Poh PE, Aryal R (2016) Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater. J Clean Prod 118:197–209
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Adsorption of congo red by three Australian kaolins. Appl Clay Sci 43:465–472
Radoor S, Karayil J, Jayakumar A, Parameswaranpillai J, Siengchin S (2021) Release of toxic methylene blue from water by mesoporous silicalite-1: characterization, kinetics and isotherm studies. Appl Water Sci 11:1–12
Wang S, Zhu Z (2006) Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J Hazard Mater 136:946–952
Namasivayam C, Muniasamy N, Gayatri K, Rani M, Ranganathan K (1996) Removal of dyes from aqueous solutions by cellulosic waste orange peel. Biores Technol 57:37–43
Namasivayam C, Radhika R, Suba S (2001) Uptake of dyes by a promising locally available agricultural solid waste: coir pith. Waste Manage 21:381–387
Wang S, Soudi M, Li L, Zhu Z (2006) Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater. J Hazard Mater 133:243–251
Khan TA, Ali I, Singh VV, Sharma S (2009) Utilization of fly ash as lowcost adsorbent for the removal of methylene blue, malachite green and rhodamine B dyes from textile wastewater. J Environ Prot Sci 3:11-22T
Khan A, Singh V, Kumar D (2004) Removal of some basic dyes from artificial textile wastewater by adsorption on Akash kinari coal. J Sci Ind Res 63:355–364
Khan TA, Sharma S, Ali I (2011) Adsorption of Rhodamine B dye from aqueous solution onto acid activated mango (Magnifera indica) leaf powder: equilibrium, kinetic and thermodynamic studies. J Toxicol Environ Health Sci 3(10):286–297
Acknowledgements
Authors gratefully thanks the Centre of Innovation in Design and Engineering for Manufacturing (CoI- DEM), KMUTNB, Thailand for characterization facilities to carry out this study. The study was financially supported by the King Mongkut's University of Technology North Bangkok (KMUTNB), Thailand through the Post-Doctoral Program (Grant Nos. KMUTNB-64-Post-03 and KMUTNB-63-Post-03 to SR)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radoor, S., Karayil, J., Jayakumar, A. et al. Adsorption of Cationic Dye onto ZSM-5 Zeolite-Based Bio Membrane: Characterizations, Kinetics and Adsorption Isotherm. J Polym Environ 30, 3279–3292 (2022). https://doi.org/10.1007/s10924-022-02421-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02421-5