Abstract
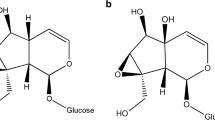
The introduction of exotic plants, animals, and pathogens into non-native ecosystems can have profound effects on native organisms. Plantago lanceolata, narrow-leaf or ribwort plantain (Plantaginaceae), is a weed that was introduced to North America from Eurasia approximately 200 years ago and that has been incorporated into the diet of a variety of native North American herbivores. Plantain contains two iridoid glycosides, aucubin and catalpol, that can be toxic or deterrent to non-specialized herbivores or herbivores that have recently incorporated this species into their diet. Anartia jatrophae (Nymphalidae), the white peacock, feeds on plants in five families including the Plantaginaceae, and was recently observed feeding on plantain; however, the effects of feeding on this novel host plant are unknown. In this study, we performed a series of experiments to assess larval preference and performance on the introduced P. lanceolata and on a native host plant that does not contain iridoid glycosides, water hyssop, Bacopa monnieri (Plantaginaceae). We also tested whether or not white peacocks were able to sequester iridoid glycosides and compared this ability with an iridoid specialist, the buckeye, Junonia coenia (Nymphalidae). White peacocks successfully developed to the adult stage on plantain; larvae grew more slowly but pupae were heavier when compared with larvae and pupae reared on the native host plant. Larvae showed induced feeding preferences for the host plant on which they were reared. Furthermore, larvae sequestered small amounts of iridoids that were also retained in pupae and adults. These results suggest that incorporation of the introduced weed, plantain, into the diet of the white peacock may have important consequences for larval performance and preference, as well as for interactions with natural enemies.




Similar content being viewed by others
References
Arany AM, de Jong TJ, Kim HK, van Dam NM, Choi YH, Verpoorte R, van der Meijden E (2008) Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 18:65–71
Belofsky G, Bowers MD, Janzen S, Stermitz F (1989) Iridoid glycosides of Aureolaria flava and their sequestration by Euphydryas phaeton butterflies. Phytochemistry 28:1601–1604
Biere A, Marak HB, van Damme JMM (2004) Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia 140:430–441
Biro PA, Abrahams MV, Post JR, Parkinson EA (2006) Behavioural trade-offs between growth and mortality explain evolution of submaximal growth rates. J Anim Ecol 75:1165–1171
Blau PA, Feeny P, Contardo L, Robson DS (1978) Allylglucosinolate and herbivorous caterpillars: a contrast in toxicity and tolerance. Science 200:1296–1298
Bowers MD (1984) Iridoid glycosides and host-plant specificity in larvae of the Buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 10:1567–1577
Bowers MD (1991) The Iridoid Glycosides. In: Rosenthal G, Berenbaum M (eds) Herbivores: Their interactions with secondary plant metabolites. Academic, New York, pp 297–327
Bowers MD (1992) The evolution of unpalatability and the cost of chemical defense in insects. In: Roitberg BD, Isman MB (eds) Insect chemical ecology: An evolutionary approach. Chapman & Hall, New York, pp 216–244
Bowers MD, Collinge SK (1992) Fate of iridoid glycosides in different life stages of the Buckeye, Junonia coenia (Lepidoptera, Nymphalidae). J Chem Ecol 18:817–831
Bowers MD, Farley S (1990) The behavior of Gray Jays, Perisoreus canadensis, towards palatable and unpalatable Lepidoptera. Anim Behav 39:699–705
Bowers MD, Puttick GM (1988) Response of generalist and specialist insects to qualitative allelochemical variation. J Chem Ecol 14:319–334
Bowers MD, Puttick GM (1989) Iridoid glycosides and insect feeding preferences: gypsy moths (Lymantria dispar, Lymantriidae) and buckeyes (Junonia coenia, Nymphalidae). J Chem Ecol 14:247–256
Bowers MD, Stamp NE (1997) Effect of hostplant genotype and pred- ators on iridoid glycoside content of pupae of a specialist insect herbivore, Junonia coenia (Nymphalidae). Biochem Syst Ecol 25:571–580
Carroll SP (2007) Natives adapting to invasive species: ecology, genes, and the sustainability of conservation. Ecol Res 22:892–901
Casagrande RA, Dacey JE (2007) Monarch butterfly oviposition on swallow-worts (Vincetoxicum spp.). Environ Entomol 36:631–636
Cavers PB, Bassett IJ, Crompton CW (1980) The biology of Canadian weeds. 47. Plantago lanceolata L. Can J Plant Sci 60:1269–1282
Chew FS (1975) Coevolution of Pierid butterflies and their cruciferous food plants. 1.Relative quality of available resources. Oecologia 20:117–127
Cory JS, Hoover K (2006) Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol 21:278–286
Daniel M (2006) Medicinal plants chemistry and properties. Science Publishers, New Hampshire
Devries PJ (1987) The butterflies of Costa Rica and their natural history. Princeton University Press, Princeton
Dyer LA, Bowers MD (1996) The importance of sequestered iridoid glycosides as a defense against an ant predator. J Chem Ecol 22:1527–1539
Harvey JA, van Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31:287–302
Harvey JA, Bukovinszky T, van der Putten WH (2010) Interactions between invasive plants and insect herbivores: a plea for a multitrophic perspective. Biol Conserv 143:2251–2259
Holeski LM, Hillstrom ML, Whitham TG, Lindroth RL (2012) Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 170:695–707
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Keeler MS, Chew FS (2008) Escaping an evolutionary trap: preference and performance of a native insect on an exotic invasive host. Oecologia 156:559–568
Kooiman P (1970) Occurrence of iridoid glycosides in Scrophulariaceae. Acta Bot Neer 19:329–340
Kooiman P (1972) Occurrence of iridoid glycosides in Labiatae. Acta Bot Neer 21:417–427
Kooiman P (1975) Occurrence of iridoid glycosides in Dicotyledoneae. 6. Occurrence of iridoid glycosides in Verbenaceae. Acta Bot Neer 24:459–468
Kuiper PJC (1992) Plantago: A multidisciplinary study. Springer, Berlin
Li Y, Zhao Y, Zhang YM, Wang MJ, Sun WJ (2009) X-ray crystal structure of iridoid glucoside aucubin and its aglycone. Carbohydr Res 344:2270–2273
Mangel M, Stamps J (2001) Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res 3:583–593
McNeely JA (2006) As the world gets smaller, the chances of invasion grow. Euphytica 148:5–15
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci U S A 98:5446–5451
Pankoke H, Bowers MD, Dobler S (2010) Influence of iridoid glycoside containing host plants on midgut β-glucosidae activity in a polyphagous caterpillar, Spilosoma virginica Fabricus (Arctiidae). J Insect Physiol 56:1907–1912
Pascual ME, Slowing K, Carretero E, Mata DS, Villar A (2001) Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol 76:201–214
Pereyra PC, Bowers MD (1988) Iridoid glycosides as oviposition stimulants for the Buckeye butterfly, Junonia coenia (Nymphalidae). J Chem Ecol 14:917–928
Puttick GM, Bowers MD (1988) Effect of the qualitative and quantitative variation in allelochemicals on a generalist insect—iridoid glycosides and the Southern Armyworm. J Chem Ecol 14:335–351
Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW, Hernandez LM (2002) Hostplants of the moth and butterfly caterpillars of America North of Mexico. The American Entomological Institute, Gainesville
Ronsted N, Gobel E, Franzyk H, Jensen SR, Olsen CE (2000) Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. Phytochemistry 55:337–348
Salah AM, Dongmo AB, Kamanyi A, Bopelet M, Vierling W, Wagner H (2000) In vitro purgative effect of Ruellia praetermissa. Sceinf. ex. Lindau (Acanthaceae). J Ethnopharmacol 72:269–272
Schoonhoven LM, Meerman J (1978) Metabolic cost of changes in diet and neutralization of allelochemics. Entomol Exp Appl 24:689–693
Scott JA (1986) The butterflies of North America a natural history and field guide. Standford University Press, Standford
Siemann E, Rogers WE, Dewalt SJ (2006) Rapid adaptation of insect herbivores to an invasive plant. Proc R Soc B Biol Sci 273:2763–2769
Silberglied RE, Aiello A, Lamas G (1979) Neotropical butterflies of the genus Anartia: systematics, life histories and general biology (Lepidoptera: Nymphalidae). Psyche 89:219–260
Singer MC, Stireman JO (2003) Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 100:554–562
Singer MC, Thomas CD, Parmesan C (1993) Rapid human-induced evolution of insect-host associations. Nature 366:681–683
Smilanich AM, Vargas J, Dyer LA, Bowers MD (2011) Effects of ingested secondary metabolites on the immune response of a polyphagous caterpillar Grammia incorrupta. J Chem Ecol 37:239–245
Staley JT, Stewart-Jones A, Poppy GM, Leather SR, Wright DJ (2009) Fertilizer affects the behaviour and performance of Plutella xylostella on brassicas. Agric For Entomol 11:275–282
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:354–371
Theodoratus DH, Bowers MD (1999) Effects of sequestered iridoid glycosides on prey choice of the prairie wolf spider, Lycosa carolinensis. J Chem Ecol 25:283–295
Verhoeven KJF, Biere A, Harvey JA, van der Putten WH (2009) Plant invaders and their novel natural enemies: who is naïve? Ecol Lett 12:107–117
Wahlberg N, Brower AVZ, Nylin S (2005) Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera : Nymphalidae). Biol J Linn Soc 86:227–251
Wurst S, van der Putten WH (2007) Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores. Basic Appl Ecol 8:491–499
Acknowledgments
We thank Evan Lampert, Barbara Demmig-Adams, Susan Whitehead, Jack Darcy, Anastasia Maines, and the members of the University of Colorado Plant-Animal Interactions Discussion group for comments on an earlier version of the manuscript and figure guidance. This study was funded by a REU Supplement to NSF grant DEB 0614883 to M.D. Bowers and L.A. Dyer and by the University of Colorado Undergraduate Research Opportunities Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knerl, A., Bowers, M.D. Incorporation of an Introduced Weed into the Diet of a Native Butterfly: Consequences for Preference, Performance and Chemical Defense. J Chem Ecol 39, 1313–1321 (2013). https://doi.org/10.1007/s10886-013-0355-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0355-3