Abstract
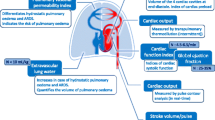
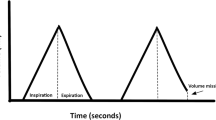
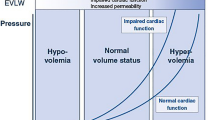
Plethysmographic signal using pulse oximetry may be used to assess fluid status of patients during surgery as it resembles arterial pressure waveform. This will avoid placement of invasive arterial lines. This study was designed to find out whether intravascular volume changes induced by mannitol bolus in neurosurgical patients are detected by variations in arterial pressure and plethysmographic waveforms and also to assess the strength of correlation between different variables derived from these two waveforms. The time difference between the onset of arterial and plethysmographic waveforms as means of significant hemodynamic changes was also evaluated. Forty one adult ASA I and II neurosurgical patients requiring mannitol infusion were recruited. Arterial line and plethysmographic probe were placed in the same limb. Digitized waveforms were collected before, at the end, and 15, 30 and 60 min after mannitol infusion. Using MATLAB, the following parameters were collected for three consecutive respiratory cycles,—systolic pressure variation (SPV), pulse pressure variation (PPV), plethysmographic peak variation (Pl-PV), plethysmographic amplitude variation (Pl-AV) and blood pressure-plethysmographic time lag (BP-Pleth time lag). Changes in above parameters over the study period were studied using repeated measure analysis of variance. Correlation between the parameters was analysed. SPV and Pl-PV showed significant increase at 15, 30 and 60 min compared to end of mannitol infusion (P < 0.01 for SPV; P < 0.05 for Pl-PV). PPV and Pl-AV showed significant increase only at 30 min (P < 0.05). The correlation between ∆SPV–∆Pl-PV, ∆PPV–∆Pl-AV and ∆SPV–∆BP-Pleth time lag were significant (r = 0.3; P < 0.01). SPV and time lag had no significant interaction. Pl-PV correlates well with SPV following mannitol infusion and can be used as an alternative to SPV. (BP-Pleth) time-lag promises to be an important parameter in assessing the state of peripheral vascular resistance and deserves further investigation.





Similar content being viewed by others
References
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–21.
Partridge BL. Use of pulse oximetry as a noninvasive indicator of intravascular volume status. J Clin Monit. 1987;3:263–8.
Bendjelid K. The pulseoximetry plethysmographic curve revisited. Curr Opin Crit Care. 2008;14:348–53.
Shamir M, Eidelman LA, Floman Y, Kaplan L, Pizov R. Pulse oximetry plethysmographic waveform during changes in blood volume. Br J Anaesth. 1999;82:178–81.
Cannesson M, Besnard C, Durand PG, Bohe J, Jacques D. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care. 2005;9:R562–8.
Lansverk SA, Hoiseth LO, Kvandal P, Hisdal J, Skare O, Kirkeboen KA. Poor agreement between respiratory variation in pulse oximetry photoplethysmographic waveform amplitude and pulse pressure in intensive care unit patients. Anesthesiology. 2008;109:849–55.
Sabharwal N, Umamaheswara Rao GS, Zulfiqar A, Radhakrishnan M. Hemodynamic changes after administration of mannitol measured by a noninvasive cardiac output monitor. J Neurosurg Anesthesiol. 2009;21:248–52.
Durga P, Jonnavittula N, Radhakrishnan M, Gopinath R. Measurement of systolic pressure variation during graded volume loss using simple tools on Datex ohmeda S/5 monitor. J Neurosurg Anesthesiol. 2009;21:161–4.
Grocott MPW, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106.
Jelsma LF, McQueen JD. Effect of experimental water restriction on brain water. J Neurosurg. 1967;26:35–40.
Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–8.
Perel A. Assessing fluid responsiveness by the systolic pressure variation in mechanically ventilated patients. Anesthesiology. 1998;89:1310–2.
Ornstein E, Eidelman LA, Drenger B, Elami A, Pizov R. Systolic pressure variation predicts the response to acute blood loss. J Clin Anesth. 1998;10:137–40.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8.
Coriat P, Vrillon M, Perel A, Baron JF, LeBret F, Saada M, Viars P. A comparison of systolic blood pressure variations and echocardiographic estimates of end-diastolic left ventricular size in patients after aortic surgery. Anesth Analg. 1994;78:46–53.
Vieillard-Baron A, Cherguri K, Augarde R, Prin S, Page B, Beaushet A, Jardin F. Cyclic changes in arterial pulse during respiratory support revisited by Doppler echocardiography. Am J Respir Crit Care Med. 2003;168:671–6.
Conflict of interest
None of the authors has any conflict of interest in the material under consideration for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radhakrishnan, M., Mohanvelu, K., Veena, S. et al. Pulse-plethysmographic variables in hemodynamic assessment during mannitol infusion. J Clin Monit Comput 26, 99–106 (2012). https://doi.org/10.1007/s10877-012-9339-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-012-9339-z