Abstract
In critically ill patients, fluid infusion is aimed at increasing cardiac output and tissue perfusion. However, it may contribute to fluid overload which may be harmful. Thus, volume status, risks and potential efficacy of fluid administration and/or removal should be carefully evaluated, and monitoring techniques help for this purpose. Central venous pressure is a marker of right ventricular preload. Very low values indicate hypovolemia, while extremely high values suggest fluid harmfulness. The pulmonary artery catheter enables a comprehensive assessment of the hemodynamic profile and is particularly useful for indicating the risk of pulmonary oedema through the pulmonary artery occlusion pressure. Besides cardiac output and preload, transpulmonary thermodilution measures extravascular lung water, which reflects the extent of lung flooding and assesses the risk of fluid infusion. Echocardiography estimates the volume status through intravascular volumes and pressures. Finally, lung ultrasound estimates lung edema. Guided by these variables, the decision to infuse fluid should first consider specific triggers, such as signs of tissue hypoperfusion. Second, benefits and risks of fluid infusion should be weighted. Thereafter, fluid responsiveness should be assessed. Monitoring techniques help for this purpose, especially by providing real time and precise measurements of cardiac output. When decided, fluid resuscitation should be performed through fluid challenges, the effects of which should be assessed through critical endpoints including cardiac output. This comprehensive evaluation of the risk, benefits and efficacy of fluid infusion helps to individualize fluid management, which should be preferred over a fixed restrictive or liberal strategy.
Similar content being viewed by others
In critically ill patients, fluid management ranges from restoring fluid depletion in hypovolemic shock, to optimization of cardiac preload to improve tissue perfusion, and fluid removal in patients with fluid overload. This review identifies how the different hemodynamic monitoring techniques help to individualize fluid management, which should be preferred to a fixed restrictive or liberal strategy |
Introduction
In critically ill patients, fluid management ranges from restoring fluid depletion in hemorrhagic shock, to optimization of cardiac preload to improve tissue perfusion in sepsis or in high-risk surgery, and fluid removal in patients with fluid overload.
In experimental sepsis, fluid administration prolongs survival [1]. However, fluids should be administered at the correct dose (defined as volume, fluid type and rate of infusion), as both insufficient and excessive volumes of fluids are associated with an increased mortality [2]. In septic patients, current guidelines suggest the initial administration of at least 30 mL/kg of crystalloids within 3 h [3]. While this formula may be valid for many patients, individualizing fluid therapy is probably preferable in some patients, especially for the sickest individuals in whom the association between amounts of fluids administered and mortality is more pronounced [4]. Tailoring fluid strategy should consider the severity of illness as well as cardiac function and other factors influencing the tolerance to fluids [5].
Fluid management varies according to the different stages of shock, with fluid administration occurring mostly during salvage and optimization phases, while fluid removal is often considered during stabilization and de-escalation phases [6]. At each stage, management should be informed by monitoring devices which may help indicate the presence of hypovolemia and need for fluids, the response to fluid infusion and the need for fluid removal.
Physiological concepts of volume status and fluid therapy
Assessing volume status covers several physiological concepts. Intravascular volume can be defined in two distribution states: a larger unstressed volume which, though present within the vascular space, does not result in a measurable vascular distending pressure (affecting vessel geometry below stretching) and a vascular stressed volume which distends the vessels and results in an increase in mean systemic pressure (Pms) [7]. Since all vascular beds have a variable volume requirement to reach their unstressed volume before creating a measurable Pms, blood flow distribution among vascular beds markedly alters the proportion of blood in the stressed and unstressed compartments. For example, the splanchnic circulation has a large unstressed volume, moving blood from splanchnic to central compartments to maintain Pms in the early (compensated) phase of hypovolemia. On the other hand, the decrease in vascular tone (sepsis, anesthesia,…) markedly decreases Pms even though total blood volume is preserved. Hence, total blood volume may be increased due to blood pooling, but the patient may still be fluid responsive. Accordingly, it is often more useful to estimate fluid responsiveness and fluid tolerance, to guide fluid administration or withdrawal.
What do we expect from fluid infusion?
The primary goal of fluid infusion is to increase cardiac output (CO), while the increase in arterial pressure is variable and depends on arterial elastance [8]. Basically, fluid infusion increases Pms, the upstream pressure of venous return. This leads to a significant increase in venous return and CO only if the ventricles are preload responsive, as in this case, Pms increases more than the right atrial pressure [9, 10]. However, estimating Pms at the bedside is not performed routinely. As the response to volume is inconstant, a key purpose of hemodynamic monitoring devices should be to help in assessing fluid responsiveness and, once infused, to assess fluid effectiveness.
Fluids may have undesired effects!
Fluid resuscitation may contribute to fluid overload. The enlargement of the vascular bed due to vasodilation, capillary leakage, and the transient hemodynamic effects of fluid boluses [11] explain why fluid infusion must be frequently repeated. In addition, maintenance fluids contribute to a large part to the total amount of fluid administered, especially after the initial optimization phase [12]. Accordingly, it will also be important to pay attention to non-resuscitative fluids, especially in patients in whom the benefits of fluid infusion are minimal.
Hypervolemia increases the intravascular pressures which promotes edema [13]. The increase in pulmonary artery pressure also contributes to right ventricular failure. The increase in central venous pressure (CVP) may impair organ perfusion pressure either directly (mean arterial pressure—CVP) or by increasing interstitial pressure (mean arterial pressure—interstitial pressure). Excess of fluids may contribute to intraabdominal hypertension or the impairment in kidney function [14].
The harmful effects of fluid overload are clearly recognized [15, 16]. Of note, cohort studies can hardly separate causation (excessive fluids inducing harm) from association (sicker patients receiving more fluids). Trials randomizing patients to restrictive versus liberal fluid resuscitation have failed to demonstrate differences in outcome [17], but most were deemed to fail as these were based on fixed regimen rather than individualizing fluid administration according to patient requirements.
In summary, the effectiveness of fluids is inconsistent, and the potentially deleterious effects are serious. Thus, hemodynamic monitoring should serve to assess both the beneficial and harmful effects of fluid therapy.
Monitoring techniques
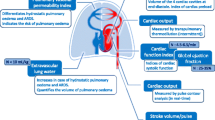
Existing monitoring techniques evaluate volume status by estimating volumes and pressures, as well as the potential consequences of excess volume such as extravascular lung water (EVLW), and venous stasis. None of these variables exactly defines volume status, and all can be affected by cardiac function, vascular permeability, and intrathoracic pressures. Furthermore, they explore different aspects of volume status and may offer complementary information when combined (Fig. 1).
Interrelation of intravascular pressures, extravascular lung water and venous stasis indices according to volume status. The relationship between intravascular pressures and volume is curvilinear and affected by cardiac function. Occurrence of extravascular lung water (EVLW) or venous stasis is dependent on volume status but may be precipitated at lower volume status in presence of impaired cardiac function, increased permeability or increased intrathoracic pressures
Estimation of cardiac preload is the cornerstone of measuring volume status. Assessing cardiac preload before and after fluid infusion is important, as it reflects the efficacy, and the risk of fluid infusion. Physiologically, ventricular preload depends on the end-diastolic pressure, volume, and ventricular compliance. Since only ventricular dimensions and pressure are used as preload markers in clinical practice, the bedside estimates of cardiac preload are all imperfect. Given the curvilinear relationship between end-diastolic pressures and volumes, volumetric measurements are more sensitive for detecting low volume states whereas pressure measurements are more sensitive for detecting hypervolemia. The relationship between pressures and volumes is shifted upwards, and its slope is steeper, in patients with poor diastolic function so that given volume changes are associated with larger increases in pressure.
Detecting hypervolemia is also essential. During the resuscitation phase, signs of hypervolemia may discourage fluid administration, even if there are signs of preload responsiveness. In the de-escalation phase, they may trigger fluid removal.
To detect hypervolemia, cardiac preload indices can be used. Importantly, presence of edema does not exclude a need for fluids [13]. Likewise, an increase in fluid balance is not systematically accompanied by an increase in blood volume, other variables should thus be considered. Measurement of EVLW and indices of venous stasis might be useful.
Plasma and blood volume measurements
Plasma volume was historically measured using different dyes or estimated from changes in hematocrit [18, 19]. While these measurements allow characterization of total blood volume, the relationship between effective circulating volume and total blood volume is inconsistent due to concomitant compensatory mechanisms (venous constriction in hypovolemia or dilation in inflammatory states) so that a patient may be fluid responsive (and hence fluids potentially indicated) regardless of total blood volume. Measurements of thoracic blood volume by bioimpedance/bioreactance are discussed in ESM.
Central venous pressure
CVP is often used to guide fluid resuscitation in two different ways [20]. First, CVP may be considered as a marker of the volume status. However, the link between CVP and volume status is not straightforward, as CVP is also influenced by venous compliance, intrathoracic and pericardial pressures, and cardiac function. Second, CVP might be used for indicating preload responsiveness, even though the ability of CVP to predict the response of CO to fluid has been challenged [21]. Extreme values can only be helpful for this purpose. A low CVP suggests that volume status is low or normal, and that fluid administration is likely to be well tolerated. On the other hand, a high CVP suggests that volume status is high or normal, or that the right ventricle is failing, and that fluid administration is potentially harmful. Median values of CVP (7-15 mmHg) are less informative [22].
Changes in CVP are also informative: increases in CVP without improvement in CO indicate that fluids are not tolerated [23]. Although far from perfect, CVP should be understood as a complex but informative variable, and its routine measurement in patients with shock should be promoted while acknowledging its limitations.
Pulmonary artery catheter
The pulmonary artery catheter (PAC) combines left and right intravascular pressure with CO measurements, allowing a comprehensive characterization of the hemodynamic profile [24]. Measurements of right ventricular volumes are discussed in ESM.
The pulmonary artery occlusion pressure (PAOP) may help to guide fluid resuscitation. Analogous to CVP, the prediction of fluid responsiveness by PAOP has been challenged [21], except for extreme values. Additionally, PAOP may help assessing the risk of lung edema induced by fluid infusion, even though the threshold at which edema occurs depends on vascular remodeling and capillary permeability.
PAOP measurements are influenced by intrathoracic pressure, however, PAOP can be corrected for it [25], and transmural PAOP is only influenced by volume status and cardiac function.
The measurement of CO by the PAC is reliable but intermittent. The “semi-continuous” measurements reflect average CO values of the preceding 3–5 min. Thus, the PAC is not suitable for evaluation of fluid responsiveness with tests that are detailed below. On the contrary, PAC provides a comprehensive and reliable assessment of tissue oxygenation with mixed venous oxygenation and carbon dioxide-derived indices, that are less reliably measured in the central venous blood.
Transpulmonary thermodilution
Transpulmonary thermodilution allows measurement of intrathoracic blood volumes, EVLW and CO [26]. Intrathoracic blood volumes represent mostly the volume of heart cavities and hence can be used as a valid surrogate of cardiac preload (detailed in ESM). As any static marker of preload, these measurements do not adequately reflect preload responsiveness. Volumetric measurements are not good to detect hypervolemia, but this is improved by coupling their analysis with CVP.
EVLW reflects the extent of lung flooding and is an independent predictor of mortality [27]. EVLW may be elevated due to increased intravascular pressure at the site of lung filtration or to increased lung capillary permeability. The increase in intravascular pressure can be related to cardiac dysfunction or to increased central blood volume. The combination of volumetric, EVLW and CVP measurements can be helpful to separate the different options (Fig. 2).
Integrative interpretation of volume status and extravascular lung water measurements. Volume status can be estimated by volumetric, pressure, or combination of both measurements. Extravascular lung water (EVLW) can be measured either by transpulmonary thermodilution, lung ultrasounds or even estimated by X-rays
Another advantage of transpulmonary thermodilution devices is the calibrated estimation of CO with pulse contour analysis which is perfect for performing tests of fluid responsiveness, like the passive leg raising (PLR) test or the end-expiratory occlusion test.
Echocardiography
Echocardiography can estimate intravascular volumes and pressures, as well as CO and function [28]. It can also identify fluid responsiveness by different indices [29]. In critically ill patients, echocardiography may rapidly identify hemodynamic phenotypes [30, 31]. It is particularly useful in patients with cardiac mechanical support. Also, it can identify patients with volume overload (Fig. 3). An important advantage of echocardiography is the easy identification of acute cor pulmonale, where fluid administration is contraindicated [32].
Echocardiography is thus an excellent tool to evaluate volume status. An important limitation of echocardiography is that estimation of filling pressures is not very precise [33] and is better suited for semi-quantitative or sequential measurements. Also, transthoracic echocardiography may be limited by poor echogenicity in some patients. Another limitation is the intermittent, rather than continuous nature of hemodynamic evaluation.
Lung ultrasound techniques
Lung ultrasound techniques do not evaluate volume status but rather the degree of lung edema [34]. B-lines indicate the presence of interstitial lung edema, but quantification is not always easy. Lung echography can also be performed in sequential manner to detect the development of lung edema by counting the number of B-lines during fluid administration [35] or weaning from mechanical ventilation [36].
Venous ultrasound techniques
Venous ultrasound evaluates the degree of venous congestion (Fig. 3). It combines estimation of the diameter of inferior vena cava (and its respiratory variations) with flow patterns in hepatic veins, portal vein and, eventually, renal veins [37]. Indices of venous stasis may be observed in hypervolemia but also in impaired right ventricular function or conditions with elevated intrathoracic pressures. While this approach has mostly been reported after cardiac surgery [38], recent data suggest that it can also be effective in other patient cohorts [39]. Sonographic evaluation of femoral veins may also be useful [40], as recently reported in patients affected by coronavirus disease 2019 (COVID-19) with right ventricular dysfunction [41].
How to integrate measurements of blood volume, EVLW and fluid responsiveness?
It is relevant to consider the patient’s illness and to combine measurements to accurately assess the hemodynamic profile. A patient may have a normal blood volume but an increased EVLW as the result of increased vascular permeability while another patient may present with an increased EVLW associated with hypervolemia. The different patterns that can be identified using combined measurements of blood volume and EVLW are presented in Fig. 2.
In addition, a patient may still benefit from fluid administration despite the presence of some degree of lung or peripheral edema. As the risk benefit profile may not be advantageous in these patients, it is crucial to determine whether these patients will be fluid responsive prior to the administration of fluids. It may also be interesting to consider more specific thresholds for fluid responsiveness in these patients [29].
When is fluid resuscitation indicated?
Several prerequisites thus need to be fulfilled. First, there should be a trigger for fluid administration (i.e. signs of tissue hypoperfusion) for which the increase in CO induced by fluid administration is considered to be a potential solution. Second, a careful evaluation of potential benefits and risks for fluids should be made. Finally, after these two first steps only, fluid responsiveness should be evaluated [42].
Selection of the trigger is crucial. Ideally, it should be an index of tissue hypoperfusion that rapidly responds to therapy (the detailed impact of fluids on tissue perfusion are reported in ESM). Prolonged capillary refill time, skin mottling, decreased venous oxygen saturation, and increased veno-arterial PCO2 gradients are excellent triggers for fluid resuscitation. Increased lactate levels are not sufficient in isolation, as hyperlactatemia may take time to resolve and may be also affected by other factors. Continuing resuscitation efforts in patients who normalized their perfusion indices was associated with worse survival [43].
A low blood pressure is often used as a trigger for fluid resuscitation [44], but the pressure response is highly variable in patients in vasodilatory state who increase their CO after fluid administration [8].
The benefit/risk balance of fluids needs to consider the amount of fluid that has already been administered (a positive response is less likely to occur if the patient has already received several liters of fluid) and the potential risks (right ventricular dysfunction, severe hypoxemia, venous congestion and intra-abdominal hypertension).
When there is an indication based on an appropriate trigger and a potentially positive benefit/risk ratio, then fluid responsiveness should be evaluated prior to fluid administration whenever feasible.
How to predict fluid responsiveness?
Due to the variability in the slope of the Frank-Starling curve, single values of markers of cardiac preload do not indicate preload responsiveness, except at high and low values. In contrast, a dynamic approach consists in observing the effects on CO, or its surrogates, of spontaneous or induced changes in cardiac preload [45]. A comprehensive review of the dynamic tests and indices of fluid responsiveness can be found elsewhere [45]. We will rather focus on how these tests benefit from hemodynamic monitoring devices. They can be separated into two categories, methods that mobilize an endogenous amount of fluid mimicking a fluid challenge, and those using variations in cardiac preload induced by mechanical ventilation.
Methods mimicking fluid challenge
While the simplest method to detect preload responsiveness is to administer a fluid bolus and measure its effect on CO, this technique can lead to fluid overload if boluses are repeated. The PLR test reproduces the hemodynamic effects of approximately 300 mL of fluid load, while being reversible [45]. Importantly, the effects of PLR cannot be reliably judged by observing changes in blood pressure or even pulse pressure which is best related to stroke volume.
Initially, the effects of the PLR test were assessed with techniques reliably measuring CO (esophageal Doppler, echocardiography, pulse wave/contour analysis) [46]. In intubated patients, end-tidal carbon dioxide may also assess changes in CO during PLR and fluid infusion, provided that ventilation is stable [47, 48].
Bioreactance may adequately detect changes in CO during PLR, provided that appropriate versions of the software are used [49], but these results require further validation. The effects of PLR may also be measured as changes in plethysmography signal amplitude, provided that vasomotor tone does not change simultaneously [50]. Echocardiography can also be used for this purpose [51]. One important limitation of these alternative measurements is reduced precision. Indeed, the changes in CO during PLR should be larger than the least significant change of the technique [52]. Accordingly, more precise techniques might be more suitable, such as pulse wave contour analysis.
Tests and indices using heart–lung interactions
Pulse pressure and stroke volume variation
Cyclic variations in stroke volume during ventilation may reflect preload responsiveness. Several indices have been reported to reflect respiratory variations in stroke volume. Arterial pulse pressure variations (PPV) were first used [53]. Most bedside monitors display PPV measurements. The essential limitation of PPV is that it cannot be used in many clinical circumstances that create false positives (spontaneous ventilation, cardiac arrhythmia, right ventricular failure) and false negatives (low tidal volume, low lung compliance, very high respiratory rate) [54]. The tidal volume challenge [55] circumvents the limits of PPV in the event of a tidal volume < 8 mL/kg [56]. It consists of increasing tidal volume transiently from 6 to 8 mL/kg and measuring the simultaneous changes in PPV [55]. A sigh maneuver can also be used in pressure support ventilation [57]. Theoretically, these tests may lead to false positive results in acute cor pulmonale which should thus be excluded by echocardiography.
Techniques that assess stroke volume beat-by-beat, such as pulse wave analysis and echocardiography can be used to assess stroke volume variations. Unavoidable measurement errors outweigh the benefit of direct estimation of stroke volume, so that PPV is preferred in adults. In children, due to the low elastance of the vessels, stroke volume variations performs better than PPV [58].
Respiratory occlusion tests
The respiratory occlusion test consists of interrupting mechanical ventilation for a few seconds and measuring the CO response. The effects of the test are difficult to measure on pulse pressure because the variations are weak and transient. Initially, this test was described with CO measured by pulse wave contour analysis [59].
The diagnostic threshold of the end-expiratory occlusion test is low (5% increase in CO), close to the smallest change detectable by many CO measurement techniques. When echocardiography is used, adding an end-inspiratory pause (which decreases CO in preload dependance) to the end-expiratory pause (which increases CO) increases the diagnostic threshold, reducing the impact of an error in the measurement of the velocity time integral [60]. Changes in the perfusion index of the plethysmography signal may also detect the effects of end-tidal occlusion [61].
Respiratory variations in vena cava
Respiratory variations of vena cava size reflect respiratory changes in venous return [62]. Respiratory variations in superior (SVC) and inferior vena cava (IVC) diameters can easily be estimated by echocardiography [29]. Initially described in mechanically ventilated patients [63], the IVC variations were also applied in spontaneously breathing patients but performance was worse, and cut-offs higher than traditionally assumed had to be used [64, 65]. The diagnostic prediction of fluid responsiveness of respiratory variations of SVC is superior to those of IVC [29], but SVC requires the use of transesophageal echocardiography. Given its limitations, IVC variations should be used in conjunction with other methods.
How to perform fluid challenge
Once the likelihood of a significant response of CO to fluid has been ascertained, the effects of volume expansion should be tested using a fluid bolus. The fluid challenge is the safest way to administer fluids. The technique was described more than 40 years ago by Max Harry Weil and refined more recently [23]: a small volume of fluid is given in a short period of time, safety limits are predefined, and critical endpoints for evaluation are settled.
Recent studies have helped to better delineate the way a fluid challenge should be assessed, and this has important consequences regarding the techniques used for hemodynamic monitoring. Regarding the volume that should be administered, evaluating the changes in CO after administration of 4 mL/kg of crystalloids over 10 min allows the identification of the maximal number of fluid responders [66], compared to slower rates or smaller amounts of fluids [66, 67]. Ideally, the effects of a fluid bolus should be assessed on CO or surrogates. Other variables such as heart rate or arterial pressure often fail to identify some CO responders. Importantly, measurements should be obtained at the end of fluid infusion, as the effects may vanish 5–10 min after the end of infusion.
Regarding the safety limits, CVP is one of the most commonly used indices [44]. Interpretation of a fluid challenge should consider changes in preload, best tracked by changes in CVP. It is usually accepted that a positive fluid challenge corresponds to an increase in CO by 10% or more with minimal changes in CVP, a negative fluid challenge to an absence of change in CO despite an increase in CVP by 3 mmHg and an indefinite response to a non-significant change in CO coupled with a non-significant change in CVP [20]. Tolerance to fluids may also take into account some other factors such as lung edema or venous stasis (Fig. 4).
Optimized fluid management. The optimal fluid management is based on defining the indication (trigger), predicting fluid responsiveness and evaluating the response to fluids both in terms of increase in perfusion but also taking into account tolerance to fluids. CRT capillary refill time, CO cardiac output, CVP central venous pressure, EVLW lung edema (estimated by various ways including transpulmonary thermodilution or lung ultrasounds, VS venous stasis
The mini-fluid challenge consists of the administration of 50–100 mL crystalloids over 1 min, to predict a subsequent response to a larger bolus [68, 69]. While the mini fluid challenge may limit fluid administration, this maneuver should be considered with caution. First, the initial bolus of fluid may not predict the response to the subsequent bolus, due to the curvilinear aspect of the Starling relationship. Second, the amount of fluid may be insufficient to elicit changes in preload and hence in CO, leading to a false negative response.
In summary, the fluid challenge technique should be standardized. A small amount of fluid is given in a short period of time, evaluating the initial response in terms of increases in stroke volume and CO, the tolerance to fluids during the administration and the dissipation of the initial effect [23].
Which strategy for fluid management?
Instead of using a fixed fluid regimen, being restrictive or liberal, it may be more appropriate to individualize fluid management according to patient’s condition and hemodynamic measurements. This approach combines the potential benefits of fluids and prevent useless administration of fluids.
Individualization of fluid management consists of giving the appropriate dose of fluid during the salvage and stabilization phases [6], refraining from fluid infusion in patients with no preload responsiveness. Fluids should also be avoided in patients in whom the risk of fluid infusion seems too high. The degree of vasodilation may also be taken into account, as an early administration of noradrenaline, which increases the stressed blood volume [70], may potentiate the effects of fluid, and help reducing the amount of fluid infused [71].
During the de-escalation phase [6], fluid removal should be adapted to the patient’s hemodynamic status. Indeed, the most important risk of fluid removal is that it exceeds its goal, and that the reduction in central blood volume is excessive, decreasing CO and blood pressure. In a recent randomized trial [72], the achieved negative fluid balance was well below the predefined target, demonstrating that patient’s condition limited the feasibility of achieving a negative fluid balance. During forced fluid removal, the rate of fluid removal had to be decreased or even stopped in 12% of the patients [73]. Both insufficient [74] and excessive rates [75] of fluid removal have been associated with increased mortality, indicating the need to individualize this therapy. What could be the relevant warning factors to predict poor tolerance to fluid removal? Excluding patients with preload responsiveness prior to fluid removal may be an option to select candidates for safe fluid removal [76] but further studies are needed to better characterize the variables that should be used to indicate and stop forced fluid removal.
Conclusions
Fluid management should be individualized. Monitoring tools are useful for all stages of fluid management from the resuscitation phase where they may be used to assess volume status, to the de-escalation phase, where they may be used to guide fluid removal. A combination of techniques is recommended to characterize the hemodynamic profiles. The choice of technique to identify fluid responsiveness is dependent on patient condition and should also be personalized.
References
Hollenberg SM, Dumasius A, Easington C, Colilla SA, Neumann A, Parrillo JE (2001) Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164:891–895
Brandt S, Regueira T, Bracht H, Porta F, Djafarzadeh S, Takala J, Gorrasi J, Borotto E, Krejci V, Hiltebrand LB et al (2009) Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care 13(6):R186
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47:1181–1247
Roberts RJ, Miano TA, Hammond DA, Patel GP, Chen JT, Phillips KM, Lopez N, Kashani K, Qadir N, Cairns CB et al (2020) Evaluation of vasopressor exposure and mortality in patients with septic shock. Crit Care Med 48(10):1445–1453
Vincent JL, Singer M, Einav S, Moreno R, Wendon J, Teboul JL, Bakker J, Hernandez G, Annane D, de Man AME et al (2021) Equilibrating SSC guidelines with individualized care. Crit Care 25(1):397
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369(18):1726–1734
Perner A, De Backer D (2014) Understanding hypovolaemia. Intensive Care Med 40(4):613–615
Monge García MI, Gil Cano A, Gracia Romero M (2011) Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care 15(1):R15
Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monnet X (2015) Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care 19:411
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, Rhodes A (2013) Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med 39(7):1299–1305
Nunes T, Ladeira R, Bafi AT, de Azevedo LCP, Machado FR, Freitas FGR (2014) Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care 4:25
Mitchell KH, Carlbom D, Caldwell E, Leary PJ, Himmelfarb J, Hough CL (2015) Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc 12(12):1837–1844
Vincent JL, Pinsky MR (2018) We should avoid the term “fluid overload.” Crit Care 22(1):214
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D (2013) Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care 17(6):R278
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34(2):344–353
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12(3):R74
Meyhoff TS, Møller MH, Hjortrup PB, Cronhjort M, Perner A, Wetterslev J (2020) Lower vs higher fluid volumes during initial management of sepsis: a systematic review with meta-analysis and trial sequential analysis. Chest 157(6):1478–1496
Awad S, Dharmavaram S, Wearn CS, Dube MG, Lobo DN (2012) Effects of an intraoperative infusion of 4% succinylated gelatine (Gelofusine(R)) and 6% hydroxyethyl starch (Voluven(R)) on blood volume. Br J Anaesth 109(2):168–176
Menth-Meier SE, Imoberdorf R, Regli B, Kipfer B, Turgay M, Ballmer PE (2001) Determination of plasma volume by indocyanine green–validation of the method and use in patients after cardiopulmonary bypass. Intensive Care Med 27:925–929
De Backer D, Vincent JL (2018) Should we measure the central venous pressure to guide fluid management? Ten answers to 10 questions. Crit Care 22(1):43
Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL (2007) Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 35(1):64–68
Biais M, Ehrmann S, Mari A, Conte B, Mahjoub Y, Desebbe O, Pottecher J, Lakhal K, Benzekri-Lefevre D, Molinari N et al (2014) Clinical relevance of pulse pressure variations for predicting fluid responsiveness in mechanically ventilated intensive care unit patients: the grey zone approach. Crit Care 18(6):587
Vincent JL, Cecconi M, De Backer D (2020) The fluid challenge. Crit Care 24(1):703
De Backer D, Hajjar LA, Pinsky MR (2018) Is there still a place for the SwanGanz catheter? We are not sure. Intensive Care Med 44(6):960–962
Teboul JL, Pinsky MR, Mercat A, Anguel N, Bernardin G, Achard JM, Boulain T, Richard C (2000) Estimating cardiac filling pressure in mechanically ventilated patients with hyperinflation. Crit Care Med 28:3631–3636
Monnet X, Teboul JL (2017) Transpulmonary thermodilution: advantages and limits. Crit Care 21(1):147
Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, Teboul JL, Monnet X (2013) Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med 42(2):472–480
De Backer D, Cholley B, Slama M, Vieillard-Baron A, Vignon P (2011) Hemodynamic monitoring using echocardiography in the critically ill. Springer, Heidelberg
Vignon P, Repesse X, Begot E, Leger J, Jacob C, Bouferrache K, Slama M, Prat G, Vieillard-Baron A (2016) Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 195:1022–1032
Lafon T, Appert A, Hadj M, Bigrat V, Legarcon V, Claveries P, Goudelin M, Evrard B, Padilla ACH, Baisse A et al (2020) Comparative early hemodynamic profiles in patients presenting to the emergency department with septic and nonseptic acute circulatory failure using focused echocardiography. Shock 53(6):695–700
Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S, Repessé X, Vieillard-Baron A (2019) Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 45(5):657–667
Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G et al (2018) Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med 44(6):774–790
Mercado P, Maizel J, Marc J, Beyls C, Zerbib Y, Zogheib E, Titeca-Beauport D, Joris M, Kontar L, Riviere A et al (2021) Doppler echocardiographic indices are specific but not sensitive to predict pulmonary artery occlusion pressure in critically ill patients under mechanical ventilation. Crit Care Med 49(1):e1–e10
Zieleskiewicz L, Muller L, Lakhal K, Meresse Z, Arbelot C, Bertrand PM, Bouhemad B, Cholley B, Demory D, Duperret S et al (2015) Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med 41(9):1638–1647
Le Bastard Q, Javaudin F, Montassier E, Pes P, Arnaudet I, Le Conte P (2020) Fluid loading guided by cardiac and lung ultrasound for patients with sepsis in the emergency department: proof-of-concept study. Eur J Emerg Med 27(3):228–229
Ferre A, Guillot M, Lichtenstein D, Meziere G, Richard C, Teboul JL, Monnet X (2019) Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med 45(5):601–608
Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY (2020) Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 12(1):16
Beaubien-Souligny W, Benkreira A, Robillard P, Bouabdallaoui N, Chassé M, Desjardins G, Lamarche Y, White M, Bouchard J, Denault A (2018) Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: a prospective observational cohort study. J Am Heart Assoc 7(19):e009961
Bhardwaj V, Vikneswaran G, Rola P, Raju S, Bhat RS, Jayakumar A, Alva A (2020) Combination of inferior vena cava diameter, hepatic venous flow, and Portal Vein Pulsatility Index: Venous Excess Ultrasound Score (VEXUS Score) in predicting acute kidney injury in patients with cardiorenal syndrome: a prospective cohort study. Indian J Crit Care Med 24(9):783–789
Denault AY, Aldred MP, Hammoud A, Zeng YH, Beaubien-Souligny W, Couture EJ, Jarry S, Gebhard CE, Langevin S, Lamarche Y et al (2020) Doppler interrogation of the femoral vein in the critically ill patient: the fastest potential acoustic window to diagnose right ventricular dysfunction? Crit Care Explor 2(10):e0209
Malinovska A, Arslani K, Zellweger N, Gebhard C, Beaubien-Souligny W, Calderone A, Siegemund M, Aschwanden M, Denault A, Gebhard CE (2021) Femoral and popliteal venous Doppler during prone and supine position in COVID-19 patients: a potential diagnostic tool to detect abnormal right ventricular function. Can J Anaesth 68(5):737–739
De Backer D (2017) Detailing the cardiovascular profile in shock patients. Crit Care 21(Suppl 3):311
Kattan E, Hernández G, Ospina-Tascón G, Valenzuela ED, Bakker J, Castro R (2020) A lactate-targeted resuscitation strategy may be associated with higher mortality in patients with septic shock and normal capillary refill time: a post hoc analysis of the ANDROMEDA-SHOCK study. Ann Intensive Care 10(1):114
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della RG, Aldecoa C, Artigas A, Jog S et al (2015) Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med 41(9):1529–1537
Monnet X, Shi R, Teboul JL (2022) Prediction of fluid responsiveness What’s new? Ann Intensive Care 12(1):46
Monnet X, Marik P, Teboul JL (2016) Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 42(12):1935–1947
Monnet X, Bataille A, Magalhaes E, Barrois J, Le Corre M, Gosset C, Guerin L, Richard C, Teboul JL (2013) End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med 39(1):93–100
Toupin F, Clairoux A, Deschamps A, Lebon JS, Lamarche Y, Lambert J, Fortier A, Denault AY (2016) Assessment of fluid responsiveness with end-tidal carbon dioxide using a simplified passive leg raising maneuver: a prospective observational study. Can J Anaesth 63(9):1033–1041
Galarza L, Mercado P, Teboul JL, Girotto V, Beurton A, Richard C, Monnet X (2018) Estimating the rapid haemodynamic effects of passive leg raising in critically ill patients using bioreactance. Br J Anaesth 121(3):567–573
Beurton A, Teboul JL, Gavelli F, Gonzalez FA, Girotto V, Galarza L, Anguel N, Richard C, Monnet X (2019) The effects of passive leg raising may be detected by the plethysmographic oxygen saturation signal in critically ill patients. Crit Care 23(1):19
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL (2007) Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med 33(7):1125–1132
Jozwiak M, Mercado P, Teboul JL, Benmalek A, Gimenez J, Depret F, Richard C, Monnet X (2019) What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care 23(1):116
Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, Teboul JL (1999) Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med 159:935–939
Teboul JL, Monnet X, Chemla D, Michard F (2019) Arterial pulse pressure variation with mechanical ventilation. Am J Respir Crit Care Med 199(1):22–31
Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL (2017) The changes in pulse pressure variation or stroke volume variation after a “tidal volume challenge” reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med 45(3):415–421
De Backer D, Heenen S, Piagnerelli M (2005) koch M, Vincent JL: Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31(4):517–523
Messina A, Colombo D, Barra FL, Cammarota G, De Mattei G, Longhini F, Romagnoli S, DellaCorte F, De Backer D, Cecconi M et al (2019) Sigh maneuver to enhance assessment of fluid responsiveness during pressure support ventilation. Crit Care 23(1):31
Durand P, Chevret L, Essouri S, Haas V, Devictor D (2008) Respiratory variations in aortic blood flow predict fluid responsiveness in ventilated children. Intensive Care Med 34(5):888–894
Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL (2009) Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med 37(3):951–956
Depret F, Jozwiak M, Teboul JL, Alphonsine JE, Richard C, Monnet X (2019) Esophageal Doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit Care Med 47(2):e96–e102
Beurton A, Gavelli F, Teboul JL, De Vita N, Monnet X (2021) Changes in the plethysmographic perfusion index during an end-expiratory occlusion detect a positive passive leg raising test. Crit Care Med 49(2):e151–e160
Magder S (2004) Clinical usefulness of respiratory variations in arterial pressure. Am J Respir Crit Care Med 169(2):151–155
Barbier C, Loubieres Y, Schmit C, Hayon J, Ricome JL, Jardin F, Vieillard-Baron A (2004) Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 30(9):1740–1746
Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY (2012) Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care 16(5):R188
Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, Ammenouche N, Seydi A, Tinturier F, Lobjoie E et al (2015) Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care 19:400
Messina A, Palandri C, De Rosa S, Danzi V, Bonaldi E, Montagnini C, Baino S, Villa F, Sala F, Zito P et al (2021) Pharmacodynamic analysis of a fluid challenge with 4 ml kg(−1) over 10 or 20 min: a multicenter cross-over randomized clinical trial. J Clin Monit Comput. https://doi.org/10.1007/s10877-021-00756-3
Aya HD, Rhodes A, Chis SI, Fletcher N, Grounds RM, Cecconi M (2017) Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med 45(2):e161–e168
Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, Zoric L, Suehs C, de la Coussaye JE, Molinari N et al (2011) An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology 115(3):541–547
Messina A, Lionetti G, Foti L, Bellotti E, Marcomini N, Cammarota G, Bennett V, Saderi L, Sotgiu G, Della Corte F et al (2021) Mini fluid chAllenge aNd End-expiratory occlusion test to assess flUid responsiVEness in the opeRating room (MANEUVER study): a multicentre cohort study. Eur J Anaesthesiol 38(4):422–431
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, Monnet X (2012) Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 40(12):3146–3153
Ospina-Tascón GA, Hernandez G, Alvarez I, Calderón-Tapia LE, Manzano-Nunez R, Sánchez-Ortiz AI, Quiñones E, Ruiz-Yucuma JE, Aldana JL, Teboul JL et al (2020) Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care 24(1):52
Silversides JA, McMullan R, Emerson LM, Bradbury I, Bannard-Smith J, Szakmany T, Trinder J, Rostron AJ, Johnston P, Ferguson AJ et al (2022) Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the Role of Active Deresuscitation After Resuscitation-2 (RADAR-2) randomised clinical trial. Intensive Care Med 48(2):190–200
Ganter CC, Hochuli R, Bossard M, Etter R, Takala J, Uehlinger DE, Jakob SM (2012) Forced fluid removal in critically ill patients with acute kidney injury. Acta Anaesthesiol Scand 56(9):1183–1190
Murugan R, Balakumar V, Kerti SJ, Priyanka P, Chang CH, Clermont G, Bellomo R, Palevsky PM, Kellum JA (2018) Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care 22(1):223
Murugan R, Kerti SJ, Chang CH, Gallagher M, Clermont G, Palevsky PM, Kellum JA, Bellomo R (2019) Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the randomized evaluation of normal vs augmented level (RENAL) of renal replacement therapy trial. JAMA Netw Open 2(6):e195418
Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, Anguel N, Richard C, Teboul JL (2016) The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 6(1):46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
DDB: Edwards Lifesciences, Philips, Baxter Nadia Aissaoui No conflict of interest. MC: Edwards Lifesciences, Directed Systems. MSC: Edwards Lifesciences. AD: Edwards Lifesciences, Masimo. LH: No conflict of interest. GH: No conflict of interest. AM: Vygon, Edwards, Philips and Getinge. SM: No conflict of interest. MO: Fresenius Medical, Baxter, Biomerieux. MRP: Edwards LifeSciences, Baxter, Intelomed, Exostat. J-LT: Getinge. PV: Baxter. J-LV: No conflict of interest. XM: Gettinge.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Backer, D., Aissaoui, N., Cecconi, M. et al. How can assessing hemodynamics help to assess volume status?. Intensive Care Med 48, 1482–1494 (2022). https://doi.org/10.1007/s00134-022-06808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-022-06808-9