Abstract
Hexagonal boron nitride (HBN), an artificial material with unique properties, is used in many industries. This article focuses on the extent to which hexagonal boron nitride and silica nanoparticles (MSN) affect the physicochemical and mechanical properties and antimicrobial activity of prepared dental composites. In this study, HBN, and MSN were used as additives in dental composites. 5% and 10% by weight of HBN are added to the structure of the composite materials. FTIR analysis were performed to determine the components of the produced boron nitride powders, hexagonal boron nitride-containing composites, and filling material applications. The structural and microstructural properties of dental composites have been extensively characterized using X-ray diffractometry (XRD). Surface morphology and distributions of nano boron nitride were determined by scanning electron microscopy (SEM)-EDS. In addition, the solubility of dental composites in water and their stability in water and chemical solution (Fenton) were determined by three repetitive experiments. Finally, the antimicrobial activity of dental composites was detected by using Minimum Inhibitory Concentration (MIC) measurement, as well as Minimum Fungicidal Concentration (MFC) method against yeast strain Saccharomyces cerevisiae, and Minimum Bactericidal Concentration (MBC) method against bacteria strains, Staphylococcus aureus and Escherichia coli. Since the HMP series have better antimicrobial activity than the HP series, they are more suitable for preventing dental caries and for long-term use of dental composites. In addition, when HMP and HP series added to the composite are compared, HMP-containing dental composites have better physicochemical and mechanical properties and therefore have a high potential for commercialization.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Dental composite refers to a filling material employed in dentistry to address areas of tooth damage. Typically white in color, dental composite blends seamlessly with the natural color of the tooth, thereby offering a visually appealing and natural appearance upon application [1, 2]. Another term used interchangeably with dental composites is resin-based material. These polymeric dental composites typically comprise a resin matrix dispersant segment and a reinforcement segment containing materials such as metal oxides, resin, and fibers for added strength and durability [3]. Composite dental resins have good durability in the dental environment regarding wear, erosion, and color stability. For this reason, it is frequently used in dental applications [4,5,6]. Dental resin composites typically have four main components: organic polymer matrix, inorganic fillers, binders such as organosilanes, and the initiator system [7, 8]. Among these components, the properties of inorganic fillers offer the advantage of controlling the strength, abrasion resistance and thermal expansion coefficient, hardness, color stability, and the softening tendency of the composite [9, 10]. One of the problems with resins is the formation of micro-cracks on the surface of dental composites through which bacteria or fungi can infiltrate [11]. The growth of microorganisms here causes caries formation and gum problems [12]. Composites designed to overcome these problems are expected to have antibacterial and antifungal properties [13, 14].
Dental caries represents a pathological deterioration of dental tissues instigated by microorganisms. Four essential conditions must converge for dental caries to manifest: the presence of a susceptible tooth surface, the presence of microorganisms within dental plaque on the tooth surface, the availability of fermentable carbohydrates, and a duration of time [15]. Furthermore, dental caries may progressively develop due to the combined effects of saliva and mechanical factors. Initially, the oral epithelium's innate immune system and salivary fluid regulate the oral microbial flora. However, alterations in salivary composition over time, coupled with surface abrasion from chewing, can contribute to caries formation. Micro and nano-sized fillers are widely employed in the production of dental Composites. Presently, various composite formulations, including micro-filled, nano-filled, micro-hybrid-filled, nanohybrid-filled, and micro-nanohybrid-filled dental composites, are favored based on the structure and size of dental caries [16, 17]. The research suggests that the antimicrobial properties of hexagonal boron nitride (HBN) and mesoporous silica nanoparticles (MSN) utilized in this study offer potential solutions to mitigate this issue [12, 18].
Glycidyl methacrylate (GMA) is a versatile and low-cost commercial monomer [19]. It also presents a pendant epoxy group that is inert to radical polymerization, allowing further functionalization of the (co)polymer obtained via nucleophilic ring-opening reactions. These functional groups offer direct applicability for immobilizing biopolymers such as enzymes, antibodies, and cells, as well as other delicate compounds. They can also undergo modification through cationic, anionic, chelating, or fluorescent pathways, broadening their versatility for diverse applications [20]. Porous silica nanoparticles (MSN) have been increasingly studied in the applications of dental materials due to their associated porous structure, mechanical properties, and good biocompatibility [21, 22]. For example, Wang et al. found that porous silica has a mechanical strengthening ability in dental resins [23]. Bai et al. prepared Zn-doped mesoporous silica nanoparticles as functional fillers, improving dental composites' mechanical and antibacterial properties [24]. Chen et al. have shown that the surface morphology of porous fillers determines the filler-resin matrix interfacial interaction and the properties of composites [25]. Compared with surface porous fillers with uniform pore size and porosity, composites containing porous silica (meso-silica) nanoparticles provided better mechanical properties and increased biocompatibility [26] .
Hexagonal boron nitride, an artificial material not found in nature, is produced by combining boron and nitrogen [27]. When hexagonal boron nitride is converted to cubic boron nitride under temperature and pressure, it has properties similar to diamond hardness [9]. HBN nanoparticles are two-dimensional nanostructures with outstanding physicochemical and biomedical properties, including excellent biocompatibility, antioxidant activity, and low toxicity [28,29,30]. Due to their excellent biocompatibility and strong mechanical and chemical stability [31, 32], HBN nanoparticles have attracted significant interest from researchers in recent years due to their potential use in clinical applications [33,34,35]. In this study, HBN known for its exceptional stability in crystal structure compared to other types of boron nitride, was the preferred choice. The crystalline structure of boron nitride is similar to carbon. For this reason, hexagonal boron nitride is often called white graphite or white carbon [36, 37]. Furthermore, HBN has found widespread utility as a functional filler within diverse cosmetic applications. Its small particle size plays a pivotal role by providing a heightened surface area, thereby augmenting the capacity to encapsulate cosmetic actives effectively. The chemical inertness, high sublimation temperature, low tangent loss, low dielectric constant, and thermal conductivity properties of hBN nanostructures, as well as their superior thermal shock resistance, are significant for composite systems. Sudden thermal shock induces void formation due to stress at composite interfaces, potentially resulting in cracks and leakage in dental composites. Thus, the incorporation of hBN structures plays a pivotal role in enhancing the thermal properties of the composite [38, 39].
Additionaly, the exceptional dispersion performance and its non-toxic, chemically inert attributes render hexagonal boron nitride a viable and advantageous ingredient in cosmetics [40, 41]. Moreover, the utilization of HBN in nanomedicine is endorsed owing to its commendable biocompatibility and biodegradability properties. Its white color and biocompatibility resolve the issue of composite coloration commonly encountered in dental composites. The study by Santosh et al. showed that HBN nanoparticles interact with bacterial cell membranes, causing cell damage and rupture, and exhibit distinct antibacterial properties [42]. Kıvanç et al. also reported that HBN nanoparticles were highly active against bacteria and fungi [40]. When applied at appropriate concentrations, they effectively impede biofilm growth by inhibiting bacterial proliferation. These findings suggest the efficacy of hexagonal boron nitride nanoparticles against oral pathogens, making them a promising choice for dental application.
Wear analysis studies concerning dental resin composites are notably scarce. In this particular investigation, the focus was primarily on exploring physical, mechanical, chemical, biological, thermal, and dynamic mechanical analyses, with wear analysis not included in the scope of the study. The wear of dental resin composites is influenced by several common factors: including temperature, composition, shear rate, normal load, elastic modulus, and hardness [43]. It is anticipated that the challenges associated with wear and other related issues can be mitigated through the utilization of HBN, MSN, and PGMA as investigated in this study.
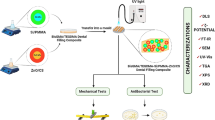
In this study, dental composites were synthesized using new HBN-containing antimicrobial materials. In addition, the characterization and antibacterial and antifungal properties of synthesized inorganic materials and composite resins were investigated.
Material and Method
Chemical and Materials
Bisphenol A glycidyl methacrylate (Bis-GMA), Triethylene glycol dimethacrylate (TEGDMA), ethyl 4-dimethylamino benzoate (4-EDMAB), Toluene (anhydrous, 99.8%), epichlorohydrin, camphorquinone (CQ), Tetraethyl orthosilicate (≥99.0% (GC)) (TEOS), Glycidyl methacrylate (≥97.0% (GC)) (GMA), Hexadecyltrimethylammonium bromide (≥98%) (CTAB) were purchased from Sigma Aldrich. Hexagonal boron nitride (70 nm, (HBN)) was purchased from Lower Friction (M.K. IMPEX CORP). Himedia supplied the peptone, yeast extract powder, and agar powder (Mumbai, India). Escherichia coli (ATCC® PTA-5976TM) (E. coli) as Gram-negative, Staphylococcus aureus (ATCC® 25923™) (S. aureus) as Gram-positive bacterial strains, and Saccharomyces cerevisiae (YPH499) (S. cerevisiae) as yeast cells were used in all experiments.
Dental Composite Preparation
MSN nanoparticles (<50 nm) were synthesized according to the referenced articles [44,45,46]. The process of photopolymerization involving glycidyl methacrylate took place within toluene, employing CQ-EDMAB as an initiator-inhibitor. The reaction environment underwent nitrogen purging and was agitated at 80°C for a duration of 24 hours [47, 48]. Within the HP and HMP series (Table 1), the HP series incorporated HBN and Poly(glycidyl methacrylate) (PGMA), while the HMP series additionally integrated MSN nanoparticles. Initially, PGMA polymer underwent homogenization in toluene, followed by the addition of HBN at concentrations of 5% and 10% for the HP series. Conversely, for the HMP series, MSN nanoparticles were included in the same proportion as PGMA and HBN, also at 5% and 10%, and subsequently mixed using a magnetic stirrer for 24 hours at 100°C within a nitrogen-rich atmosphere. The formulation of the dental composites involved determining the proportions of inorganic and resin components based on findings from our prior research [49]. Table 1 gives information about the preparation of resins with the inorganic part of dental composites.
FTIR Spectroscopy
FTIR is a technique that provides information about the specific bonds of materials. The FTIR spectrum of HBN and composite structures was recorded in permeability mode using the Perkin Elmer Spectrum 100 FTIR Spectrometer device to determine organic components of the samples and reveal the interaction between the hexagonal nano boron nitride supplement and materials. Measurements were made at room temperature in the 4000 to 400 cm−1[46].
XRD
XRD patterns of the composites were determined with the multi-purpose Bruker D-8 Advance X-Ray Device with Cu Kα radiation [49].
SEM-EDS Analysis
The Scanning Electron Microscope (SEM-Philips XL 30 SFEG) is a versatile imaging tool that allows image formation by scanning the sample surface of high-energy electrons. In addition, in parallel with SEM examinations, the presence of other nanoparticles and their distribution in the structure were investigated by conducting elemental analysis on the applied Energy Distribution Spectroscopy (EDS-EDAX) Mapping samples [50].
TGA
Thermogravimetric analysis (TGA) is an analytical technique used to determine the thermal stability of the material and the fraction of rapidly evaporating components by recording the weight change that occurs when a sample is heated in air or a controlled atmosphere. To determine the shift in mass of the products and to obtain information about the effect of HBN on the thermal stability of inorganic parts and composites, Linseis PT 1600 Instruments model TGA located at Gebze Technical University, Department of Materials Science and Engineering Research Laboratory, was used at 10°C/1000°C between 25°C-1000°C. Analysis were carried out in a nitrogen atmosphere at a speed of 15 min [51].
DSC
DSC analysis was conducted on a dental composite containing HBN (Hexagonal Boron Nitride) to interpret the mass degradation behavior influenced by the temperature of the organic matrix and the composite during sample heating. Measurements were performed using a Netzsch DSC 404 C Pegasus instrument under a nitrogen atmosphere, with a heating rate of 10 °C up to 250 °C [49].
Chemical Stability, Water Sorption and Solubility
The Fenton reaction, used to evaluate the chemical stability of organic materials, calculates the degradation rates of the materials using hydrogen peroxide (H2O2) and iron (Fe) cations. These analysis were made with reference to our previous study (Supplementary Figure 1) [49].The water solubility-sorption of the composites and used for the ISO-4049 International Standard values (Supplementary Figure 2) [52].
Mechanical Properties
Utilizing the INSTRON 5569 compression test apparatus, mechanical properties of the dental composites were evaluated. Cylindrical samples with a thickness of 3 mm and a diameter of 5 mm were tested under axial stress with a crosshead speed of 0.2 mm/min [49].
Antimicrobial Activity of Inorganic Parts of Dental Composites
The minimum inhibition concentration (MIC) measurement was used to evaluate the antifungal and antibacterial activity of the dental composites. MIC measurements were performed for S. cerevisiae yeast cells based on the study of Konuk et al. [53]; for Gram-negative E. coli and Gram-positive S. aureus bacteria cells based on the study of Ergüden et al. [54]. Yeast or bacteria cells were treated with various amounts of HP5, HP10, HMP5, and HMP10 in microtiter plates to determine inhibition of the materials and incubated with shaking. The lowest amount of HBN nanoparticles with no visible growth of yeast or bacterial cells was accepted as the MIC value for each nanoparticle. After MIC measurements of inorganic parts of dental composites, minimum fungicidal concentration (MFC) and minimum bactericidal concentration (MBC) measurements were determined for yeast and bacteria cells, respectively. MFC or MBC is the lowest nanoparticle concentration at which no visible growth observed on agar plates [53, 55, 56]. For MFC measurement, 10 µL of yeast cells which showed no visible growth were taken from all the wells of MIC microtiter plate after 48 hours and seeded on agar plates. The agar plates were incubated at 28°C for 24 hours. For MBC measurement, 5 µL of bacterial cells was taken from all the wells after 24 hours, seeded on agar plates and incubated at 37°C for 18 hours. In all experiments, samples were prepared in triplicates for each concentration, and the experiments were repeated at least three times.
Results
FTIR spectroscopy
FTIR spectra reflect characteristic vibrations of particular bonds between particular groups of atoms and are commonly used to identify specific substances such as organic groups. The obtained spectra contain molecular fingerprints that allow easy scanning of samples containing many different components. The FTIR spectra in Figures 1 were obtained at room temperature at 4000-400 cm-1. When the FTIR graphs were examined, it was seen that 10% HBN added plates gave more profound peaks in each material group. Boron nitride nanopowders exhibit two characteristic vibration modes: in-plane axial B-N-B vibration and out-of-plane B-N vibration, found at approximately 1327 cm-1 and 764 cm-1 respectively, these peaks are not sharp but have a flat shape.
XRD
Figure 2 presents the preliminary analysis of HBN, D5, D10, DM5 and DM10.
SEM-EDS Analysis
SEM was used to examine the surface morphologies of the inorganic components and dental composites, as shown in Fig. 3 and 4. The atomic percentage table distributions obtained as a result of the EDS analysis are given in Table 2.
TGA
In Figure 5, temperature-related mass loss results of 5%, 10%, and HBN-containing composites by weight are given. It has been observed that the thermal resistance of silica-based dental composites is better than others. In addition, when the composites are compared within themselves, it is seen in the graph that the thermal resistance decreases as the HBN ratio increases.
DSC
The DSC curves of dental composites were examined, and the Tg values were observed to be between 190 and 218◦C (Figure 6 and Table 3). The increase in the HBN concentration in the content somewhat raised the Tg values when the Tg values of the nanocomposites were investigated. Due to the MSN nanoparticles they contain, DM nanocomposites were found to have greater Tg values than D nanocomposites.
Chemical stability, water sorption and solubility
Fig. 7 presents the results obtained from loss of material, water sorption and solubility of dental composites. To check the chemical stability of dental composites, they were kept in Fenton solution at 40 degrees for two days. Material losses are reduced by adding MSN to dental composites. By comparison, the most negligible material loss is seen in DM5 (1.42%) (Figure 7). Dental composites containing HBN showed water solubility and sorption behavior. However, Wsl and Wsp of dental composites are compatible with the hydrophobicity of dental composites. Water absorption and solubility in water decreased significantly with MSN added to the structure in the inorganic part. As the HBN ratio in the structure increased, the water absorption and water solubility of the HP and HMP-containing dental composites decreased. D5 and DM5 showed the lowest water solubility and sorption values. (DM5 < DM10 < D5 < ID10).
Mechanical Properties
Mechanical characteristics including elastic modulus, compression strength, and maximum stress and strain at failure are shown in Figure 8. As can be observed in Figure 8, the results indicated that DM5 (103.48 ± 3.01 MPa) had the highest average compressive strength value. The strain value at break with the greatest value was D5 (17.13 ± 0.98 MPa). However, the composite material D10 (5.91 ± 0.35 MPa) has the maximum elasticity modulus. Elastic modulus and compression strength values for hybrid-filled dental composites range from 2 to 6 MPa and 31 to 103 MPa, respectively. Additionally, DM5's average compression strength significantly outperforms dental composites created with hybrid fillings.
Antimicrobial Activity of Inorganic Parts of Dental Composites
MIC values of HP5, HP10, HMP5 and HMP10, the inorganic parts of dental composites synthesized in this study against Gram-negative bacterial strain E. coli, Gram-positive bacteria S. aureus and yeast S. cerevisiae are given in Table 4. Moreover, MBC results for bacteria strains and MFC results for the yeast strain are reported (Table 4 and Supplementary Figure 3). HP5 and HP10 have low activity against both yeast and bacteria cells (MIC: >20 mg/mL, MFC and MBC: >20 mg/mL). On the other hand, HMP5 and HMP10 were effective at different concentrations against yeast and bacteria strains. They have more activity against yeast cells compared to bacteria cells (MIC and MFC: 1-5 mg/mL for yeast cells) (Table 4).
Discussion
Characterization
The novelty of this work is to combine the unique properties of HBN and MSN nanoparticles to create chemically resistant, antimicrobial dental composites with high mechanical strength. The resulting HMP-containing dental composites will be characterized as a more economical approach that will trigger new dental restorations, have a higher mechanical strength and also provide an antimicrobial effect. FTIR spectra confirmed the synthesis of inorganic ingredients and modifications of dental composites [28, 57]. To discuss the XRD results of HBN-doped materials comparatively, the information obtained from the literature was examined in detail. The XRD results of the additives used and the composites produced are given in Figure 2. The diffraction peaks are not intense and sharp because of the dominant epoxy phase structure. In XRD models, along with the comprehensive, flat amorphous structure from epoxy matrices, (002), (100) peaks of weak characteristic HBN were observed [58]. The characteristic peak of the hexagonal boron nitride with the plane at about 26° could be partially seen when doping at a rate of 10% in the composite plates. The peak at 26.8° corresponds to the crystal plane of boron nitride (002) [59]. It is thought that composite plates, which are amorphous in general structure, suppress the existing boron nitride peaks. In addition, it was concluded that it is difficult to detect a contribution of 5 and 10% with XRD. SEM images of the nanoparticles and dental composites are given in Figure 3. In addition, EDS mapping analysis was performed for each composite group to understand the distribution of nanoparticles in epoxy matrices. As the HBN percentage increased, it decreased more per unit area; thus, it could be observed more clearly. Also, the absence of voids that could have a detrimental effect on the mechanical properties was determined by SEM analysis [40, 59]. Mapping is a type of elemental analysis made over the color chart according to the intensities of the elements in the EDS examination area. In mapping, where the whole picture is analyzed instead of a point or a selected region, all elements in the periodic table are scanned and visualized by coloring; in the EDS mapping results, dental composites contain a high percentage of carbon, and changes in the dominant atom were observed [60]. It was observed that the designed dental composites were resistant to thermal degradation. The thermal stability of the dental composite increased with the addition of mesoporous silica content to the inorganic part. This indicates that breaking the cross-links of the polymers in the structure requires much more energy [61]. Both the increase in functional nanoparticles and the addition of mesoporous silica to the structure increased the Tg values of the dental composites when compared. The inorganic part that fills the matrix and the hydrogen bonding of the silica nanoparticles with HBN and PGMA can be shown to be the reason for this [62]. When the water solubility and sorption of dental composites were examined, an increase was observed when the inorganic content of the composite was increased from 5% to 10%. For this reason, dental composites with 5% content are more advantageous than 10%. Despite the hydroxyl group in the structure of Bis-GMA-based composites, they have excessive water absorption [63]. When the inorganic part of the HMP content increases, the rate of increase in water solubility and sorption is lower than the HP content. It was also observed that the addition of MSN to the structure increased the hydrophobicity of the dental composite. This higher hydrophobicity is thought to be associated with lower water absorption, water swelling, and hydrolytic/enzymatic degradation of dental composites in the oral environment [64, 65]. The compression resistance values of dental composites increased with the addition of silica content to the structure. While the addition of 5% of HMP structure had a positive effect, the strength decreased when the ratio increased to 10%. While this mesoporous structure had a positive effect in filling the voids, the increase in the ratio in the structure had a negative effect since it is a brittle material [24, 25]. When the materials were observed with fracture points, it was observed that the brittleness increased in the structure when the inorganic content increased. This is due to the fragile structure of HBN and MSN in the content. Since the elastic properties of HBN nanoparticles are better than MSN nanoparticles, the elastic modulus of D series nanoparticles is higher than DM series [66]. The literature review scrutinized the mechanical properties of commercially available dental composites such as OMNICHROMA, Filtek Supreme Ultra, TPH Spectra, Tetric EvoCeram, and Herculite Ultra. Upon comparison with these clinical-grade products, it was noted that the designed dental composite exhibited relatively lower elastic modulus and compression stress properties [67]. Future research endeavors aim to enhance these properties by adjusting the resin-inorganic material ratio and refining processes during the polymerization stage, with the goal of achieving mechanical characteristics comparable to those of commercial products.
Antimicrobial Activity of Inorganic Parts of Dental Composites
Microbial dental plaques are the main etiological factor for periodontal disease [68,69,70]. Accumulation of microbial biofilms around the teeth induces the formation of periodontal pockets and is finalized with periodontal disease [71]. Therefore, testing the antimicrobial properties of dental materials is a crucial necessity for application. Since S. aureus and E. coli as oral pathogens produce biofilms, they are associated with dental diseases [72,73,74] . Moreover, in dental disease, yeasts also have an important role [74]. S. cerevisiae as a model organism for yeasts has a crucial relation with some oral lesions [75]. In this study, we used Gram-negative E. coli and Gram-positive S. aureus as bacterial cells, and S. cerevisiae as yeast cells to test the antibacterial and antifungal activities of HP5, HP10, HMP5 and HMP10, the inorganic parts of the dental composites. The antimicrobial activity of hexagonal boron nitride nanoparticles was tested before [40, 42]. In addition to their studies, here, we determined the antibacterial and antifungal activity of hexagonal boron nitride nanoparticles as inorganic parts of dental composites at different concentrations as a specific application and showed the effectiveness against different bacteria and yeast strains. HP5 and HP10 have lower effect on both bacteria and yeast cells based on the MIC, MBC and MFC values, which are > 20 mg/mL for all (Table 4 and Supplementary Figure 3). For E. coli, HMP5 is more effective than HMP10 (both MIC and MBC values are 1-5 mg/mL for HMP5; 5-10 mg/mL for HMP10). On the contrary, HMP10 is more active than HMP5 against S. aureus bacterial cells (MIC value is 5-10 mg/mL for HMP5 and 1-5 mg/mL for HMP10) (Table 4). Our MBC studies with S. aureus cells using the inorganic parts of HP5, HP10, HMP5 and HMP10 resulted with growth of bacteria cells at all tested concentrations and thus the MBC values for all compounds are >20 mg/mL. Surprisingly though, S. aureus cells treated with different concentrations of HMP5 or HMP10 have clear growth defects compared to the HP5 or HP10 treated cells. They can be considered as bacteriostatic against S. aureus cells because of inhibition of growth and multiplication of cells instead of killing cells at used concentration (Supplementary Figure 4). For yeast cells, both HMP5 and HMP10 have comparable antifungal effect based on the MIC and MFC values (1-5 mg/mL). In conclusion, our overall antimicrobial activity studies reveal that HMP nanoparticles are more effective against both bacterial and fungal cells than HP nanoparticles. Presumably the incorporation of porous MSN components into the composition of nanoparticles in HMPs led to the inclusion of higher amounts of active antimicrobial groups onto the nanoparticles.
Conclusion
This study compares the effects of HBN and MSN nanoparticles on dental composites. The presence of a single Tg value for the composites confirms the homogeneous structure identified in SEM images. Additionally, this may be attributed to crosslinked structure of the composites. Showing a tendency for degradation at high temperatures and possessing a high Tg value also confirms the free volume theory for composites. The lowest water absorption (0.044 mg mm-3) and solubility (0.006 mg mm-3) were achieved in the DM5. The chemical resistance of dental composites against Fenton solution was demonstrated by negligible mass loss: D5: 2.372%, DM5: 1.44%, D10: 2.457%, and DM10: 2.20%. The DM10 nanocomposite had the highest Tg value, at a temperature of 218°C, when the Tg values of the nanocomposites were examined. Dental composites with a 5% HBN content had higher compression stress and strain at break values than those with a 10% HBN content. On the other hand, the elastic modulus of dental composites from the D series increased as the HBN level did. Within the studied concentration range (1-20 mg/mL), the presence of MSN in the structure increased the surface binding of antimicrobial HBN nanoparticles, resulting in a significant enhancement of the antimicrobial property in the inorganic part of HMP5 and HMP10.
Upon evaluating all characterization and antibacterial test results, it can be said that the thermal, mechanical, and chemical durability properties of the produced composite may be comparable to currently applicable dental composites. Furthermore, the utilization of nanoparticles with high surface area in dental composites, facilitated by filler-resin interaction, has resulted in the development of dental composites with superior chemical and mechanical properties. This study also contributes to the prevention of problems commonly associated with the use of micro-sized fillers in current dental composite systems, such as the formation of microcracks and leakage, which often lead to bacterial proliferation..The high biocompatibility and unique antimicrobial properties of HBN nanostructures are innovative and significant for the dental composites included in this study. This study was conducted to determine the effects of HBN and MSN nanoparticles on the chemical, mechanical, and antimicrobial activity properties of dental composites. These experimental results are believed to contribute to the development of dental composites designed for industrial applications.
Data and Code Availability
Not Applicable
References
R. Yadav, H. Lee, J. H. Lee, R. K. Singh, and H. H. Lee (2023). A comprehensive review: Physical, mechanical, and tribological characterization of dental resin composite materials. Tribol. Int. 179, 108102. https://doi.org/10.1016/j.triboint.2022.108102.
R. Yadav, A. Meena, and A. Patnaik (2022). Biomaterials for dental composite applications: A comprehensive review of physical, chemical, mechanical, thermal, tribological, and biological properties. Polym. Adv. Technol. 33, 1762–1781. https://doi.org/10.1002/pat.5648.
R. Yadav and A. Meena (2022). Effect of aluminium oxide, titanium oxide, hydroxyapatite filled dental restorative composite materials on physico-mechanical properties. Ceram. Int. 48, 20306–20314. https://doi.org/10.1016/j.ceramint.2022.03.311.
M. Zarei, I. Mohammadzadeh, A. Derakhshani, K. Saidi, and H. Sheibani (2023). Synthesis of new dental monomers based on glycidyl methacrylate and their evaluation of cytotoxic and antibacterial activity. Polym. Test. 117, 107818. https://doi.org/10.1016/j.polymertesting.2022.107818.
Y. Sun, Z. Zhou, H. Jiang, Y. Duan, J. Li, X. Liu, L. Hong, and C. Zhao (2021). Preparation and evaluation of novel bio-based Bis-GMA-free dental composites with low estrogenic activity. Dent. Mater. 38, 1–13. https://doi.org/10.1016/j.dental.2021.12.010.
R. Yadav, M. Singh, A. Meena, S. Y. Lee, and S. J. Park (2023). Selection and ranking of dental restorative composite materials using hybrid Entropy-VIKOR method: An application of MCDM technique. J. Mech. Behav. Biomed. Mater. 147, 106103. https://doi.org/10.1016/j.jmbbm.2023.106103.
S. K. Usul, A. Aslan, H. B. Lüleci, B. Ergüden, M. T. Çöpoğlu, H. Oflaz, A. M. Soydan, and D. Özçimen (2022). Investigation of antimicrobial and mechanical effects of functional nanoparticles in novel dental resin composites. J. Dent. 123, 104180. https://doi.org/10.1016/j.jdent.2022.104180.
R. Yadav (2022). Fabrication, characterization, and optimization selection of ceramic particulate reinforced dental restorative composite materials. Polym. Polym. Compos. 30, 1–10. https://doi.org/10.1177/09673911211062755.
A. S. Alansy, T. A. Saeed, R. Al-Attab, Y. Guo, Y. Yang, B. Liu, and Z. Fan (2022). Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite. Dent. Mater. 38, e266–e274. https://doi.org/10.1016/j.dental.2022.08.010.
S. Saini, A. Meena, R. Yadav, and A. Patnaik (2023). Tribology International Investigation of physical, mechanical, thermal, and tribological characterization of tricalcium phosphate and zirconia particulate reinforced dental resin composite materials. Tribol. Int. 181, 108322. https://doi.org/10.1016/j.triboint.2023.108322.
R. Yadav and A. Meena (2022). Mechanical and two-body wear characterization of micro-nano ceramic particulate reinforced dental restorative composite materials. Polym. Compos. 43, 467–482. https://doi.org/10.1002/pc.26391.
M. Chi, M. Qi, A. Lan, P. Wang, M. D. Weir, M. A. Melo, X. Sun, B. Dong, C. Li, J. Wu, L. Wang, and H. H. K. Xu (2019). Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int. J. Mol. Sci. 20, 278. https://doi.org/10.3390/ijms20020278.
C. Montoya, L. Roldan, M. Yu, S. Valliani, C. Ta, M. Yang, S. Orrego, G. Gib, and U. Eafit (2023). Bioactive Materials Smart dental materials for antimicrobial applications. Bioact. Mater. 24, 1–19. https://doi.org/10.1016/j.bioactmat.2022.12.002.
X. He, L. Ye, R. He, J. He, S. Ouyang, and J. Zhang (2022). Antibacterial dental resin composites ( DRCs ) with synthesized bis-quaternary ammonium monomethacrylates as antibacterial agents. J. Mech. Behav. Biomed. Mater. 135, 105487. https://doi.org/10.1016/j.jmbbm.2022.105487.
B. Pratap, M. Nag, R. Yadav, S. Althahban, and J. C. Wal (2023). Dynamic mechanical analysis of zinc oxide and hydroxyapatite particulate filled dental restorative composite materials. AIP Conf. Proc. 2782, 5–10. https://doi.org/10.1063/5.0154476.
R. Yadav and M. Kumar (2020). Investigation of the physical, mechanical and thermal properties of nano and microsized particulate-filled dental composite material. J. Compos. Mater. 54, 2623–2633. https://doi.org/10.1177/0021998320902212.
M. Chi, N. Li, N. Sharma, W. Li, C. Chen, B. Dong, L. Cheng, L. Wang, and F.M. (2022). Thieringer, Positive regulation of osteogenesis on titanium surface by modification of nanosized Ca2+-exchanged EMT zeolites. Mater. Today Commun. 33, 104874. https://doi.org/10.1016/j.mtcomm.2022.104874.
M. Qi, M. Chi, X. Sun, X. Xie, M. D. Weir, T. W. Oates, Y. Zhou, L. Wang, Y. Bai, and H. H. K. Xu (2019). Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomedicine. 14, 6937–6956. https://doi.org/10.2147/IJN.S212807.
M. Faria, C. Vilela, F. Mohammadkazemi, A. J. D. Silvestre, C. S. R. Freire, and N. Cordeiro (2019). Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: Preparation, characterization and post-modification. Int. J. Biol. Macromol. 127, 618–627. https://doi.org/10.1016/j.ijbiomac.2019.01.133.
E. M. Muzammil, A. Khan, and M. C. Stuparu (2017). Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 7, 55874–55884. https://doi.org/10.1039/c7ra11093f.
H. Kong, X. Bai, H. Li, C. Lin, X. Yao, and Y. Wang (2022). Preparation of Ca doped wrinkled porous silica ( Ca-WPS ) for the improvement of apatite formation and mechanical properties of dental resins. Journal of the Mechanical Behavior of Biomedical Materials 129, 105159.
A. Aminoroaya, R. Bagheri, S. Nouri Khorasani, Z. Talebi, P. Derakhshanfar, and R. EsmaeelyNeisiany (2022). Mesoporous silica aerogel reinforced dental composite: Effects of microstructure and surface modification. J. Mech. Behav. Biomed. Mater. 125, 104947. https://doi.org/10.1016/j.jmbbm.2021.104947.
R. Wang, E. Habib, and X. X. Zhu (2017). Synthesis of wrinkled mesoporous silica and its reinforcing effect for dental resin composites. Dent. Mater. 33, 1139–1148. https://doi.org/10.1016/j.dental.2017.07.012.
X. Bai, C. Lin, Y. Wang, J. Ma, X. Wang, X. Yao, and B. Tang (2020). Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 36, 794–807. https://doi.org/10.1016/j.dental.2020.03.026.
H. Chen, H. Liu, R. Wang, X. Jiang, and M. Zhu (2021). Size-controllable synthesis of dendritic porous silica as reinforcing fillers for dental composites. Dent. Mater. 37, 961–971. https://doi.org/10.1016/j.dental.2021.02.015.
S. Saini, A. Meena, R. Yadav, and A. Patnaik (2023). Fabrication, Evaluation, and Performance Ranking of Tri-calcium Phosphate and Silica Reinforced Dental Resin Composite Materials. Silicon. 15, 8045–8063. https://doi.org/10.1007/s12633-023-02646-6.
S. C. Yoo, J. Kim, S. Kim, and D. Lee (2022). Enhanced mechanical properties of melamine-functionalized boron nitride nanosheets reinforced with epoxy nanocomposites for dental applications. J. Mater. Sci. 57, 18205–18219. https://doi.org/10.1007/s10853-022-07702-x.
P. Ahmad, A. Khalid, M. U. Khandaker, F. Rehman, M. I. Khan, H. Ali, N. Muhammad, M. S. Kiyani, A. Sulieman, M. A. Rauf Khan, Z. Razzaq, A. Khan, S. Haq, Y. Saeed, and M. I. Irshad (2022). The antibacterial and antioxidant efficacy and neutron sensing potency of 10B enriched hexagonal boron nitride nanoparticles. Mater. Sci. Semicond. Process. 141, 106419. https://doi.org/10.1016/j.mssp.2021.106419.
M. Li, G. Huang, X. Chen, J. Yin, P. Zhang, Y. Yao, J. Shen, Y. Wu, and J. Huang (2022). Perspectives on environmental applications of hexagonal boron nitride nanomaterials. Nano Today. 44, 101486. https://doi.org/10.1016/j.nantod.2022.101486.
F. W. Degrazia, V. C. B. Leitune, F. Visioli, S. M. W. Samuel, and F. M. Collares (2018). Long-term stability of dental adhesive incorporated by boron nitride nanotubes. Dent. Mater. 34, 427–433. https://doi.org/10.1016/j.dental.2017.11.024.
N. Özmeriç, G. Ö. Çakal, C. Gökmenoğlu, A. Özmeriç, B. F. Oduncuoğlu, T. Hacaloğlu, and B. Kaftanoğlu (2022). Histomorphometric and biomechanical evaluation of the osseointegration around micro- and nano-level boron-nitride coated titanium dental implants. J. Stomatol. Oral Maxillofac. Surg. 123, e694–e700. https://doi.org/10.1016/j.jormas.2022.06.016.
B. Lee, J. S. Kwon, M. W. Khalid, K. M. Kim, J. Kim, K. M. Lim, and S. H. Hong (2020). Boron nitride nanoplatelets as reinforcement material for dental ceramics. Dent. Mater. 36, 744–754. https://doi.org/10.1016/j.dental.2020.03.002.
W. Huang, D. Mei, H. Qin, J. Li, L. Wang, X. Ma, S. Zhu, and S. Guan (2022). Electrophoretic deposited boron nitride nanosheets-containing chitosan-based coating on Mg alloy for better corrosion resistance, biocompatibility and antibacterial properties. Colloids Surfaces A Physicochem. Eng. Asp. 638, 128303. https://doi.org/10.1016/j.colsurfa.2022.128303.
Z. N. Kayani, Z. Bashir, M. Mohsin, S. Riaz, and S. Naseem (2021). Sol-gel synthesized boron nitride (BN) thin films for antibacterial and magnetic applications. Optik (Stuttg). 243, 167502. https://doi.org/10.1016/j.ijleo.2021.167502.
G. Çakal, C. Gökmenoğlu, B. Kaftanoğlu, and N. Özmeriç (2019). Surface characterization and corrosion resistance of boron nitride coated titanium dental implants. Prot. Met. Phys. Chem. Surfaces. 55, 608–614. https://doi.org/10.1134/S2070205119030079.
M. Li, S. Wang, R. Li, Y. Wang, X. Fan, W. Gong, and Y. Ma (2022). The mechanical and antibacterial properties of boron nitride/silver nanocomposite enhanced polymethyl methacrylate resin for application in oral denture bases. Biomimetics. 7, 138. https://doi.org/10.3390/biomimetics7030138.
I. Mitruţ, I. R. Scorei, H. O. Manolea, A. Biţă, L. Mogoantă, J. Neamţu, L. E. Bejenaru, M. V. Ciocîlteu, C. Bejenaru, G. Rău, and G. D. Mogoşanu (2022). Boron-containing compounds in Dentistry a narrative review. Rom. J. Morphol. Embryol. 63, 477–483. https://doi.org/10.47162/RJME.63.3.01.
M. R. Abdul Karim, M. A. Khan, A. U. Zaman, and A. Hussain (2023). Hexagonal boron nitride-based composites: an overview of processing approaches and mechanical properties. J. Korean Ceram. Soc. 60, 1–23. https://doi.org/10.1007/s43207-022-00251-8.
H. Abouelleil, C. Jeanin, and B. Grosgogeat (2016). Thermal effect shock on the enamel-composite restoration interface. Br. J. Appl. Sci. Technol. 17, 1–10. https://doi.org/10.9734/bjast/2016/27343.
M. Kıvanç, B. Barutca, A. T. Koparal, Y. Göncü, S. H. Bostancı, and N. Ay (2018). Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C. 91, 115–124. https://doi.org/10.1016/j.msec.2018.05.028.
S. Unal, N. Ekren, A. Z. Sengil, F. N. Oktar, S. Irmak, O. Oral, Y. M. Sahin, O. Kilic, S. Agathopoulos, and O. Gunduz (2018). Synthesis, characterization, and biological properties of composites of hydroxyapatite and hexagonal boron nitride. J. Biomed Mater. Res. - Part B Appl. Biomater. 106, 2384–2392. https://doi.org/10.1002/jbm.b.34046.
S. Pandit, K. Gaska, V. R. S. S. Mokkapati, S. Forsberg, M. Svensson, R. Kádár, and I. Mijakovic (2019). Antibacterial effect of boron nitride flakes with controlled orientation in polymer composites. RSC Adv. 9, 33454–33459. https://doi.org/10.1039/c9ra06773f.
R. Yadav, A. Meena, H. H. Lee, S. Y. Lee, and S. J. Park (2023). Tribological behavior of dental resin composites: A comprehensive review. Tribol. Int. 190, 109017. https://doi.org/10.1016/j.triboint.2023.109017.
M. Hachemaoui, B. Boukoussa, A. Mokhtar, A. Mekki, M. Beldjilali, M. Benaissa, F. Zaoui, A. Hakiki, W. Chaibi, M. Sassi, and R. Hamacha (2020). Dyes adsorption, antifungal and antibacterial properties of metal loaded mesoporous silica: Effect of metal and calcination treatment. Mater. Chem. Phys. 256, 123704. https://doi.org/10.1016/j.matchemphys.2020.123704.
M. J. Son and S. W. Lee (2021). Antibacterial toxicity of mesoporous silica nanoparticles with functional decoration of specific organic moieties. Colloids Surfaces A Physicochem. Eng. Asp. 630, 127612. https://doi.org/10.1016/j.colsurfa.2021.127612.
S. K. Usul, H. B. Lüleci, B. Ergüden, and A. Aslan (2023). Antimicrobial properties of azole functional silica nanocomposites. ChemistrySelect. 8, 1–10. https://doi.org/10.1002/slct.202303059.
A. Aslan and A. Bozkurt (2010). Bioinspired blend membranes based on adenine and guanine functional poly(glycidyl methacrylate). Langmuir. 26, 13655–13661. https://doi.org/10.1021/la102096y.
A. Aslan, A. M. Soydan, and A. Bozkurt (2015). Synthesis and characterization of novel multifunctional polymer grafted hollow silica spheres. J. Mater. Res. 30, 2408–2416. https://doi.org/10.1557/jmr.2015.222.
S. KaptanUsul, A. Aslan, H. B. Lüleci, B. Ergüden, M. T. Çöpoğlu, H. Oflaz, A. M. Soydan, and D. Özçimen (2022). Investigation of antimicrobial and mechanical effects of functional nanoparticles in novel dental resin composites. J. Dent. 123, 104180. https://doi.org/10.1016/j.jdent.2022.104180.
S. KaptanUsul, H. B. Lüleci, N. S. Değirmenci, B. Ergüden, A. M. Soydan, and A. Aslan (2024). Differential silica nanoparticles functionalized with branched Poly(1-Vinyl-1,2,4-Triazole): antibacterial, antifungal, and cytotoxic qualities. J. Nanomater. 2024, 1–11. https://doi.org/10.1155/2024/9998736.
S. KaptanUsul, B. Binay, A. M. Soydan, and A. Aslan (2024). A newly synthesized magnetic nanoparticle coated with glycidyl methacrylate monomer and 1,2,4-Triazole: Immobilization of α-Amylase from Bacillus licheniformis for more reuse, stability, and activity in the presence of H2O2. Bioorg. Chem. 143, 107068. https://doi.org/10.1016/j.bioorg.2023.107068.
J. A. Müller, N. Rohr, and J. Fischer (2017). Evaluation of ISO 4049: water sorption and water solubility of resin cements. Eur. J. Oral Sci. 125, 141–150. https://doi.org/10.1111/eos.12339.
H. BüşraKonuk and B. Ergüden (2017). Antifungal activity of various essential oils against Saccharomyces cerevisiae depends on disruption of cell membrane integrity. Biocell. 41, 13–18. https://doi.org/10.32604/biocell.2017.41.013.
B. Ergüden (2021). Phenol group of terpenoids is crucial for antibacterial activity upon ion leakage. Lett. Appl. Microbiol. 73, 438–445. https://doi.org/10.1111/lam.13529.
A. Kunicka-Styczyńska (2011). Activity of essential oils against food-spoiling yeast. A review. Flavour Fragr. J. 26, 326–328. https://doi.org/10.1002/ffj.2046.
P. Parvekar, J. Palaskar, S. Metgud, R. Maria, and S. Dutta (2020). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus, Biomater. Investig. Dent. 7, 105–109. https://doi.org/10.1080/26415275.2020.1796674.
K. I. Nasser, J. M. Liñeira del Río, F. Mariño, E. R. López, and J. Fernández (2021). Double hybrid lubricant additives consisting of a phosphonium ionic liquid and graphene nanoplatelets/hexagonal boron nitride nanoparticles. Tribol. Int. 163, 107189. https://doi.org/10.1016/j.triboint.2021.107189.
W. Zhao, W. Zhao, Z. Huang, G. Liu, and B. Wu (2019). Tribological performances of epoxy resin composite coatings using hexagonal boron nitride and cubic boron nitride nanoparticles as additives. Chem. Phys. Lett. 732, 136646. https://doi.org/10.1016/j.cplett.2019.136646.
M. Doğan, A. Selek, O. Turhan, B. K. Kızılduman, and Z. Bicil (2021). Different functional groups functionalized hexagonal boron nitride (h-BN) nanoparticles and multi-walled carbon nanotubes (MWCNT) for hydrogen storage. Fuel. 303, 121335. https://doi.org/10.1016/j.fuel.2021.121335.
I. M. Joni, R. Balgis, T. Ogi, T. Iwaki, and K. Okuyama (2011). Surface functionalization for dispersing and stabilizing hexagonal boron nitride nanoparticle by bead milling. Colloids Surfaces A Physicochem. Eng. Asp. 388, 49–58. https://doi.org/10.1016/j.colsurfa.2011.08.007.
H. ErdemÇamurlu, S. Mathur, O. Arslan, and E. Akarsu (2016). Modification of hexagonal boron nitride nanoparticles with fluorosilane. Ceram. Int. 42, 6312–6318. https://doi.org/10.1016/j.ceramint.2016.01.019.
F. Z. Cherchali, N. Attik, M. Mouzali, J. B. Tommasino, H. Abouelleil, D. Decoret, D. Seux, and B. Grosgogeat (2020). Structural stability of DHMAI antibacterial dental composite following in vitro biological aging. Dent. Mater. 36, 1161–1169. https://doi.org/10.1016/j.dental.2020.05.006.
Z. Li, H. Zhang, G. Xiong, J. Zhang, R. Guo, L. Li, H. Zhou, G. Chen, Z. Zhou, and Q. Li (2020). A low-shrinkage dental composite with epoxy-polyhedral oligomeric silsesquioxane. J. Mech. Behav. Biomed. Mater. 103, 103515. https://doi.org/10.1016/j.jmbbm.2019.103515.
L. Cao, J. Yan, T. Luo, H. Yan, F. Hua, and H. He (2023). Antibacterial and fluorescent clear aligner attachment resin modified with chlorhexidine loaded mesoporous silica nanoparticles and zinc oxide quantum dots. J. Mech. Behav. Biomed. Mater. 141, 105817. https://doi.org/10.1016/j.jmbbm.2023.105817.
J. Thadathil Varghese, K. Cho, P. Raju, L. Farrar, and B.G. Prusty. Prentice (2023). Effect of silane coupling agent and concentration on fracture toughness and water sorption behaviour of fibre-reinforced dental composites. Dent. Mater. 39, 362–371. https://doi.org/10.1016/j.dental.2023.03.002.
A.T. Shah, S. Attique, M.A. ur Rehman, A.S. Khan, O. Goerke (2020). Silica-based antibacterial coatings for dental implants, Dental Implants, 145–171. https://doi.org/10.1016/B978-0-12-819586-4.00009-3.
M. A. Ahmed, R. Jouhar, and Z. Khurshid (2022). Smart monochromatic composite: a literature review. Int. J. Dent. 2022, 2–9. https://doi.org/10.1155/2022/2445394.
P. E. Petersen (2004). Continuous improvement of oral health in the 21st century: the approach of the WHO Global Oral Health Programme. Zhonghua Kou Qiang Yi Xue Za Zhi. 39, 441–444.
P. E. Petersen, D. Kandelman, S. Arpin, and H. Ogawa (2010). Global oral health of older people–call for public health action., Community Dent. Health. 27, 257–267. https://doi.org/10.1922/CDH.
W. Li, M. Qi, X. Sun, M. Chi, Y. Wan, X. Zheng, C. Li, L. Wang, and B. Dong (2020). Novel dental adhesive containing silver exchanged EMT zeolites against cariogenic biofilms to combat dental caries. Microporous Mesoporous Mater. 299, 110113. https://doi.org/10.1016/j.micromeso.2020.110113.
P. N. Papapanou, M. Sanz, N. Buduneli, T. Dietrich, M. Feres, D. H. Fine, T. F. Flemmig, R. Garcia, W. V. Giannobile, F. Graziani, H. Greenwell, D. Herrera, R. T. Kao, M. Kebschull, D. F. Kinane, K. L. Kirkwood, T. Kocher, K. S. Kornman, P. S. Kumar, B. G. Loos, E. Machtei, H. Meng, A. Mombelli, I. Needleman, S. Offenbacher, G. J. Seymour, R. Teles, and M. S. Tonetti (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 45, S162–S170. https://doi.org/10.1111/jcpe.12946.
A. Uribe-García, G. L. Paniagua-Contreras, E. Monroy-Pérez, J. Bustos-Martínez, A. Hamdan-Partida, J. Garzón, J. Alanís, R. Quezada, F. Vaca-Paniagua, and S. Vaca (2021). Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. J. Microbiol. Immunol. Infect. 54, 267–275. https://doi.org/10.1016/j.jmii.2019.05.010.
J. Estemalik, C. Demko, N. F. Bissada, N. Joshi, D. Bodner, E. Shankar, and S. Gupta (2017). Simultaneous Detection of Oral Pathogens in Subgingival Plaque and Prostatic Fluid of Men With Periodontal and Prostatic Diseases. J. Periodontol. 88, 823–829. https://doi.org/10.1902/jop.2017.160477.
C. Yadufashije, M. Ingabire, M. Sibomana, E. Munyeshyaka, J. Mucumbitsi, F. Niyonzima, A. Onyango Mala, G. B. Sangano, L. Mwanzia, and T. Habyarimana (2021). Association of Oral Microbial Community Dysbiosis and Dental Disorders Among pregnant woman Attending Gatenga Health Center, Kigali, Rwanda. J. Med. Sci. Heal. 7, 32–37. https://doi.org/10.46347/jmsh.2021.v07i01.006.
J. N. Algazaq, K. Akrami, F. Martinez, A. McCutchan, and A. R. Bharti (2017). Saccharomyces cerevisiae Laryngitis and Oral Lesions in a Patient with Laryngeal Carcinoma. Case Rep. Infect. Dis. 2017, 1–4. https://doi.org/10.1155/2017/2941527.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Sedef Kaptan Usul: Term, Conceptualization Ideas, Methodology, Development or design of methodology, Validation Verification, Investigation, Resources, Writing - Review and Editing, Visualization, Project administration
Hatice Büşra Lüleci: Writing - Review and Editing, Visualization, Methodology, Development or design of methodology
Bengü Ergüden: Writing - Review and Editing, Visualization, Methodology, Development or design of methodology
Ayşe Aslan Canpolat: Term, Conceptualization Ideas, Methodology, Development or design of methodology, Investigation, Resources, Writing - Review and Editing, Visualization, financial support
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable
Conflicts of İnterest or Competing İnterests
Not Applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Mesoporous nanoparticles are used in a variety of bioengineering applications.
• Hexagonal boron nitride has unique antimicrobial properties.
• The antimicrobial properties of the designed dental composite are influenced by the quantities of hexagonal boron nitride (HBN) incorporated, and also mesoporous silica.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaptan Usul, S., Aslan, A., Lüleci, H.B. et al. Effects of Hexagonal Boron Nitride and Mesoporous Silica Nanoparticles on the Morphology, Mechanical Properties and Antimicrobial Activity of Dental Composites. J Clust Sci (2024). https://doi.org/10.1007/s10876-024-02658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10876-024-02658-1