Abstract
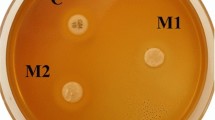
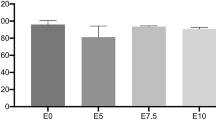
In this study, Si/PMMA and ZnO/CS core–shell structures were synthesized as inorganic fillers and added to a bisphenol A diglycidyl dimethacrylate (Bis-GMA)/triethylene glycol dimethacrylate (TEGDMA) (50%/50%) commercial dental composite at a weight percentage (wt%) of 0.1–1.0% to simultaneously improve the mechanical and antibacterial properties of the composite. The prepared composites were characterized using dynamic light scattering (DLS), zeta potential (ζ-potential), Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), ultraviolet–visible spectrophotometer (UV–Vis), thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), and X-ray powder diffraction (XRD). The mechanical properties, including flexural strength and flexural modulus, of the composite were investigated by three-point bending tests. Additionally, the antibacterial activity against the Staphylococcus aureus (S. aureus) bacteria strain was evaluated using the colony-forming unit method to assess the antibacterial effect of the novel composite. As a result, the Bis-GMA/TEGDMA-Si/PMMA/ZnO/CS dental composite, reinforced with 0.1 wt% Si/PMMA and 0.5 wt% ZnO/CS, exhibited a 46% improvement in flexural strength and a 56% improvement in flexural modulus compared to pristine Bis-GMA/TEGDMA. Moreover, the novel Bis-GMA/TEGDMA-Si/PMMA/ZnO/CS dental composite showed a 95% reduction in bacterial growth against the S. aureus bacteria strain.
Graphical Abstract










Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request from the authors.
References
Agnihotri S, Pathak R, Jha D et al (2015) Synthesis and antimicrobial activity of aminoglycoside-conjugated silica nanoparticles against clinical and resistant bacteria. New J Chem 39:6746–6755
Ahmed N, Majid A, Khan MA et al (2018) Synthesis and characterization of Zn/ZnO microspheres on indented sites of silicon substrate. Mater Sci 36:501–508
Al-Abboodi SMT, Yahya AN, Almussawy TA (2020) Preparation and characterization of chitosan/{ZnO}/Ag nanocomposites as antibacterial hydrogel for wound dressing. IOP Conf Ser Mater Sci Eng 978:12040
Al-Shamahy H, Al-labani M (2020) Prevalence of staphylococcus aureus ın dental ınfectıons and the occurrence of MRSA ın ısolates. Univ J Pharm Res 5(2):23–7
Alzraikat H, Burrow M, Maghaireh G, Taha N (2018) Nanofilled resin composite properties and clinical performance: a review. Oper Dent 43:E173–E190
Aykaç A, Akkaş EÖ (2022) Synthesis, characterization, and antibacterial properties of ZnO nanostructures functionalized flexible carbon fibers. Recent Pat Nanotechnol 17(2):119–130
Buşilă M, Muşat V, Textor T, Mahltig B (2015) Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv 5:21562–21571
Canché-Escamilla G, Duarte-Aranda S, Toledano M (2014) Synthesis and characterization of hybrid silica/PMMA nanoparticles and their use as filler in dental composites. Mater Sci Eng C 42:161–167
Cetin AR, Unlu N, Cobanoglu N (2013) A five-year clinical evaluation of direct nanofilled and indirect composite resin restorations in posterior teeth. Oper Dent 38:E31–E41
Chang Q-Q, Cui Y-W, Zhang H-H et al (2019) C-doped ZnO decorated with Au nanoparticles constructed from the metal–organic framework ZIF-8 for photodegradation of organic dyes. RSC Adv 9:12689–12695
Chen Q, Zhao Y, Wu W et al (2012) Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing halloysite nanotubes. Dent Mater 28:1071–1079
Dananjaya SHS, Kumar RS, Yang M et al (2018) Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int J Biol Macromol 108:1281–1288
De Mori A, Di Gregorio E, Kao AP et al (2019) Antibacterial PMMA composite cements with tunable thermal and mechanical properties. ACS Omega 4:19664–19675
El-Banna A, Sherief D, Fawzy AS (2019) 7 - Resin-based dental composites for tooth filling. In: Khurshid Z, Najeeb S, Zafar MS (eds) Sefat FBT-ADB. Woodhead Publishing, New Delhi, pp 127–173
Elizalde-Peña EA, Flores-Ramirez N, Luna-Barcenas G et al (2007) Synthesis and characterization of chitosan-g-glycidyl methacrylate with methyl methacrylate. Eur Polym J 43:3963–3969
Gajewski VES, Pfeifer CS, Fróes-Salgado NRG et al (2012) Monomers used in resin composites: degree of conversion, mechanical properties and water sorption/solubility. Braz Dent J 23:508–514
Gnanasangeetha D, Saralathambavani D (2013) One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res J Mater Sci 1:1–8
Haldorai Y, Shim J-J (2013) Chitosan-zinc oxide hybrid composite for enhanced dye degradation and antibacterial activity. Compos Interfaces 20:365–377
Harb SV, dos Santos FC, Pulcinelli SH et al (2016) Protective coatings based on PMMA–silica nanocomposites reinforced with carbon nanotubes. In: Berber MR, Hafez IH (eds) Carbon nanotubes. IntechOpen, Rijeka
Javed R, Rais F, Fatima H et al (2020) Chitosan encapsulated ZnO nanocomposites: fabrication, characterization, and functionalization of bio-dental approaches. Mater Sci Eng C 116:111184
Kaptan Usul S, Aslan A, Lüleci HB et al (2022) Investigation of antimicrobial and mechanical effects of functional nanoparticles in novel dental resin composites. J Dent 123:104180
Karabela MM, Sideridou ID (2011) Synthesis and study of properties of dental resin composites with different nanosilica particles size. Dent Mater 27:825–835
Khademi M, Wang W, Reitinger W, Barz DP (2017) The Zeta potential of poly PMMA in contact with aqueous electrolyte–surfactant solutions. Langmuir 33(40):10473–10482
Kim HC, Noh SM, Park SK (2013) Synthesis and characterization of nanosilica ball-PMMA hybrid composites. J Appl Polym Sci 127:1653–1658
Kumar S, Koh J (2012) Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int J Mol Sci 13:6102–6116
Kundie F, Azhari CH, Muchtar A, Ahmad ZA (2018) Effects of filler size on the mechanical properties of polymer-filled dental composites: a review of recent developments. J Phys Sci 29:141–165
Li L-H, Deng J-C, Deng H-R et al (2010) Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr Res 345:994–998
Masud RA, Islam MS, Haque P et al (2020) Preparation of novel chitosan/poly (ethylene glycol)/ZnO bionanocomposite for wound healing application: effect of gentamicin loading. Materialia 12:100785
McCormack MG, Smith AJ, Akram AN et al (2015) Staphylococcus aureus and the oral cavity: an overlooked source of carriage and infection? Am J Infect Control 43:35–37
Nguyen NT, Nguyen NT, Nguyen VA (2020) In situ synthesis and characterization of ZnO/chitosan nanocomposite as an adsorbent for removal of congo red from aqueous solution. Adv Polym Technol 2020:3892694
Petrizza L, Collot M, Richert L et al (2016) Dye-doped silica nanoparticle probes for fluorescence lifetime imaging of reductive environments in living cells. RSC Adv 6:104164–104172
Salehi R, Mahmoodi NM, Bahrami H, Khorramfar S (2010) Novel biocompatible composite (Chitosan-zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf B Biointerfaces 80:86–93
Şen Karaman D, Gulin-Sarfraz T, Hedström G et al (2014) Rational evaluation of the utilization of PEG-PEI copolymers for the facilitation of silica nanoparticulate systems in biomedical applications. J Colloid Interface Sci 418:300–310
Sodagar A, Khalil S, Kassaee MZ et al (2016) Antimicrobial properties of poly (methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J Orthod Sci 5:7–13
Song W, Ge S (2019) Application of antimicrobial nanoparticles in dentistry. Molecules 24:1033
Sundeep D, Vijaya Kumar T, Rao PSS et al (2017) Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog Biomater 6:57–66
Swain SK, Dey RK, Islam M et al (2009) Removal of fluoride from aqueous solution using aluminum-impregnated chitosan biopolymer. Sep Sci Technol 44:2096–2116
Taslı H, Akbıyık A, Topaloğlu N et al (2018) Photodynamic antimicrobial activity of new porphyrin derivatives against methicillin resistant Staphylococcus aureus. J Microbiol 56:828–837
Vega-Jiménez AL, Almaguer-Flores A, Flores-Castañeda M et al (2017) Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 28:435101
Wang H, Ren D (2017) Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express 7:204
Wilson KS, Antonucci JM (2006) Interphase structure-property relationships in thermoset dimethacrylate nanocomposites. Dent Mater 22:995–1001
Yang Y, Dan Y (2003) Preparation of PMMA/SiO2 composite particles via emulsion polymerization. Colloid Polym Sci 281:794–799
Yang Y, Xu Z, Guo Y et al (2021) Novel core–shell CHX/ACP nanoparticles effectively improve the mechanical, antibacterial and remineralized properties of the dental resin composite. Dent Mater 37:636–647
Yusof NAA, Zain NM, Pauzi N (2019) Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int J Biol Macromol 124:1132–1136
Zaharia A, Muşat V, Pleşcan Ghisman V, Baroiu N (2016) Antimicrobial hybrid biocompatible materials based on acrylic copolymers modified with (Ag)ZnO/chitosan composite nanoparticles. Eur Polym J 84:550–564
Zhang H, Li C, Guo J et al (2012) In situ synthesis of poly(methyl methacrylate)/SiO2 hybrid nanocomposites via “grafting onto” strategy based on UV ırradiation in the presence of ıron aqueous solution. J Nanomater 2012:1–9
Zhang F, Lan J, Yang Y et al (2013) Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J Nanopart Res 15:1–10
Zhao D, Yu S, Sun B et al (2018) Biomedical applications of chitosan and its derivative nanoparticles. Polymers 10:462
Acknowledgements
This work was supported by İzmir Katip Çelebi University Scientific Research Council (IKCU-BAP). The authors would like to thank the IKCU-BAP for the financial support via 2021-ÖDL-FEBE-0002 project number.
Author information
Authors and Affiliations
Contributions
All authors have reviewed and agreed to the published version of the manuscript. IO contributed to conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, and writing—review and editing. AA contributed to conceptualization, methodology, investigation, validation, formal analysis, supervision, resources, review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ok, I., Aykac, A. Enhancement of the mechanical and antibacterial properties of Bis-GMA/TEGDMA dental composite incorporated with ZnO/CS and Si/PMMA core–shell nanostructures. Chem. Pap. 77, 6959–6973 (2023). https://doi.org/10.1007/s11696-023-02989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02989-9