Abstract
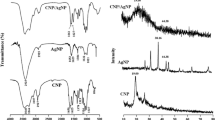
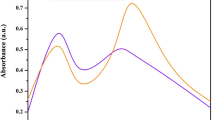
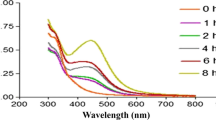
Chitosan derived silver biocomposites (CsS) were produced by green synthesis using Carmona retusa (Vahl) Masam aqueous leaf extract. UV–Vis spectra of synthesized CsS biocomposites showed absorption maxima at 441 nm. FESEM average particle size was 51 nm and spherical in shape. TEM images of CsS biocomposite ranged between 30 nm to 60 nm, the DLS measurement showed the size of 234.1 nm for chitosan derived AgNPs. In FTIR spectra, the C–H winding and were observed for CsS biocomposites. In addition, the elemental composition showed uniform grain boundaries as recognized by EDaX spectra. In-vitro antioxidant activity CsS biocomposites showed the ability to scavenge free radicals. Cytotoxicity analysis of CsS biocomposites on MCF-7 breast cancer cell line revealed 90% inhibition at 500 μg/ml concentration. C. retusa mediated synthesis AgNPs coated chitosan biocomposite showed strong larvicidal activity with low LC50 and LC90 values against the malarial vector, An. stephensi, Ae. aegypti, and Cx. quinquefasciatus respectively. Eight bioactive components were present in aqueous leaf extracts of C. retusa. Based on this study we suggest that C. retusa plant mediated AgNPs chitosan derived silver biocomposites (CsS) has anti-cancerous and insecticidal activity which can further be explored for commercialization.










Similar content being viewed by others
References
S. Arivalagan, S. Ravichandran, K. Rangasamy, and E. Karthikeyan (2011). Int. J. Chem. Tech. Res. 3, 534–538.
M. K. Teli, S. Mutalik, and G. K. Rajanikant (2010). Curr. Pharm. Des. 16, 1882–1892.
A. Domard (2011). Carbohydr. Polym. 84, 696–703.
S. Cumana, J. Simons, A. Liese, L. Hilterhaus, and I. Smirnova (2013). J. Mol. Catal. B Enzym. 85–86, 220–228.
M. Imperiyka, A. Ahmad, S. A. Hanifah, and F. Bella (2014). Phys. B 450, 151–154.
Y. Xie, J. Zhao, Z. Le, M. Li, J. Chen, Y. Gao, Y. Huang, Y. Qin, R. Zhong, D. Zhou, and Y. Ling (2014). Compos. Sci. Technol. 99, 141–146.
M. Catauro, F. Bollino, P. Veronesi, and G. Lamanna (2014). Mater. Sci. Eng. C 39, 344–351.
Y. He, X. Li, Y. Zheng, Z. Wang, Z. Ma, Q. Yang, B. Yao, Y. Zhao, and H. Zhang (2018). New J. Chem. 42, 2882–2888.
S. Sarkar, E. Guibal, F. Quignard, and A. K. SenGupta (2012). J. Nanopart. Res. 14, 715.
N. R. Abdelsalam, A. Abdel-Mageed, H. M. Ali, M. Z. M. Salem, M. F. A. Al-Hayali, and M. S. Elshikh (2018). Ecotoxicol. Environ. Saf. 155, 76–85.
K. C. Mouli, T. Vijaya, and S. D. Rao (2009). Pharm. Technol. 1, 4–8.
D. L. Shrisha, K. A. Raveesha, and N. Nagabhushan (2011). J. Med. Plants. Res. 17, 4087–4093.
R. Rajkumar, M. S. Shivakumar, S. Senthil Nathan, and K. Selvam (2018). J. Clust. Sci. 29, 1243–1253.
Y. He, F. Wei, Z. Ma, H. Zhang, Q. Yang, B. Yao, Z. Huang, J. Li, C. Zeng, and Q. Zhang (2017). RSC Adv. 7, 39842–39851.
S. Some, I. K. Sen, A. Mandal, T. Aslan, Y. Ustun, E. S. Yilmaz, A. Kati, A. Demirbas, A. K. Mandal, and I. Ocsoy (2019). Mater. Res. Express. 6, 012001–012022.
G. Benelli (2015). Parasitol. Res. 114, 2801–2805.
P. Vivekanandhan, S. Karthi, M. S. Shivakumar, and G. Benelli (2018). Pathogens Global Health 112, 37–46.
G. Benelli and R. Pavela (2018). Ind. Crops Prod. 117, 382–392.
G. Benelli (2016). Parasitol. Res. 115, 23–34.
A. Anitha, S. Sowmiya, P. T. Sudheesh Kumar, S. Deepthi, K. P. Chennazhi, H. Ehrlich, M. Tsurkan, and R. Jayakumar (2014). Prog. Polym. Sci. 39, 1644–1667.
P. Molyneux (2004). J. Sci. Technol. 26, 211–219.
B. Halliwell, J. M. Gutteridge, and O. I. Aruoma (1987). Anal. Biochem. 165, 215–219.
T. Mosmann (1983). J. Immunol. Methods 65, 55–63.
WHO (2005). cds/WHO-pes/gcdpp/13.
W. S. Abbott (1925). J. Ecol. Entomol. 18, 265–267.
P. Sivalingam, J. J. Antony, D. Siva, S. Achiraman, and K. Anbarasu (2012). Colloids Surf. B Biointerfaces 98, 12–17.
A. Maniraj, S. Muthuram Kumar, M. Kannan, K. Rajarathinam, and A. Pushparaj (2017). J. Adv. Appl. Sci. Res. 9, 97–106.
R. Vivek, R. Thangam, K. Muthuchelian, P. Gunasekaran, and K. S. Kaveri Kannan (2012). Process. Biochem. 47, 2405–2410.
K. Gopinath, S. Gowri, and A. Arumugam (2013). J. Nano Chem. 3, 68–75.
D. Wei, W. Sun, W. Qian, Y. Ye, and X. Ma (2009). Carbohydr. Res. 344, 2375–2382.
S. Dudonne, X. Vitrac, P. Coutiere, M. Woillez, and J. M. Merillon (2009). J. Agric. Food Chem. 57, 1768–1774.
I. Skandrani, J. Boubaker, W. Bhouri, I. Limem, S. Kilani, M. Ben Sghaier, A. Neffati, I. Bouhlel, K. Ghedira, and L. Chekir-Ghedira (2010). Drug Chem. Toxicol. 33, 20–27.
I. Skandrani, I. Limem, A. Neffati, J. Boubaker, M. Ben Sghaier, W. Bhouri, I. Bouhlel, S. Kilani, K. Ghedira, and L. Chekir-Ghedira (2010). Food Chem. Toxicol. 48, 710–715.
C. S. Moody and H. M. Hassan (1982). Proc. Natl. Acad. Sci. U S A 79, 2855–2859.
H. M. Hassan and I. Fridovich (1979). J. Biol. Chem. 254, 10846–10852.
C. E. Schwartz, J. Krall, L. Norton, K. McKay, D. Kay, and R. E. Lynch (1983). J. Biol. Chem. 258, 6277–6281.
H. Rosen and S. J. Klebanoff (1979). J. Exp. Med. 149, 27–39.
G. Applerot, A. Lipovsky, R. Dror, N. Perkas, Y. Nitzan, and R. Lubart (1968). Adv. Funct. Mater. 19, 842–852.
K. J. A. Davies (1968). J. Biol. Chem. 262, 9895–9901.
J. M. Gutteridge, D. A. Rowley, and B. Halliwell (1981). Biochem. J. 199, 263–265.
R. L. Baldwin (1968). J. Dairy Sci. 51, 104–111.
F. Regoli, M. Nigro, S. Bompadre, and G. W. Winston (2000). Aquat. Toxicol. 49, 13–25.
C. S. Ryu, C. H. Kim, S. Y. Lee, K. S. Lee, K. J. Choung, G. Y. Song, B. H. Kim, S. Y. Ryu, H. S. Lee, and S. K. Kim (2012). Food Chem. 132, 333–337.
S. Banerjee, J. P. Saikia, A. Kumar, and B. K. Konwar (2010). Nanotechnology 21, 045101–045108.
G. Kiran, M. Sarangapani, T. Gouthami, and A. R. Narsimha Reddy (2013). Toxic. Environ. Chem. 95, 367–378.
K. Gopinath, M. Chinnadurai, N. P. Devi, K. Bhakyaraj, S. Kumaraguru, T. Baranisri, A. Sudha, M. Zeeshan, A. Arumugam, M. Govindarajan, and N. S. Alharbi (2017). J. Clust. Sci. 28, 621–635.
J. Ching, T. K. Chua, L. C. Chin, A. J. Lau, Y. K. Pang, J. M. Jaya, C. H. Tan, and H. L. Koh (2010). Indian J. Exp. Biol. 48, 275–279.
R. Vidhya and R. Udayakumar (2015). Int. J. Biochem. Res. Rev. 7, 192–203.
F. Nikkon, K. A. Salam, T. Yeasmin, A. Mosaddik, P. Khondkar, and M. E. Haque (2010). Pharm. Biol. 48, 264–268.
G. Ramkumar, S. Karthi, R. Muthusamy, P. Suganya, D. Natarajan, E. J. Kweka, and M. S. Shivakumar (2016). PLoS ONE 11, 1–11.
Acknowledgements
This research was funded by University Grants Commission-Rajiv Gandhi National Fellowship Programme (Sanction Number: F1-17.1/2016-17/RGNF-2015-17-SC-TAM-26510) for their financial support. The authors also are thankful to the Department of Botany, School of Life Sciences, Periyar University, Salem, Tamil Nadu-636 011, India, for providing infrastructural facility, and KIRND Institute of Research and Development Pvt Ltd, Tiruchirappalli, Tamil Nadu-620 020, India, for GC–MS analysis and Antioxidant studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajkumar, R., Shivakumar, M.S., Senthil Nathan, S. et al. Preparation and Characterization of Chitosan Nanocomposites Material Using Silver Nanoparticle Synthesized Carmona retusa (Vahl) Masam Leaf Extract for Antioxidant, Anti-cancerous and Insecticidal Application. J Clust Sci 30, 1145–1155 (2019). https://doi.org/10.1007/s10876-019-01578-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01578-9