Abstract
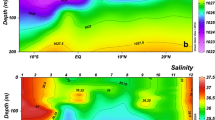
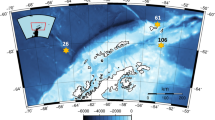
This study examined the biomass structure of autotrophic and heterotrophic plankton along a trophic gradient in the northwestern Pacific Ocean in an attempt to understand planktonic food web structure. Autotrophic biomass exceeded that of heterotrophic organisms in all sampling regions, but with lesser contribution to total planktonic biomass at stations of higher phytoplankton biomass, including the northern East China Sea, compared to the regions of lower phytoplankton biomass. The proportion of the biomass of heterotrophic bacteria, nanoflagellates (HNF), and dinoflagellates (HDF) relative to that of phytoplankton was all inversely related to phytoplankton biomass, but positive relationships were observed for both ciliates and mesozooplankton. Mesozooplankton biomass inclined greater than phytoplankton along the gradient of phytoplankton biomass, with biomass rise being most closely associated with ciliate and HDF biomass and, to a lesser degree, with large phytoplankton (>3 μm). Both bacteria and picophytoplankton were significantly and positively related to the biomass ratio of mesozooplankton to the sum of HDF and ciliates (i.e., proxy of mesozooplankton predation on protozoans), but no positive relationship was apparent either for HNF or for large phytoplankton. Such relationships may result from predation relief on lower food webs associated with mesozooplankton feeding on protistan plankton.








Similar content being viewed by others
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Ara K (2001) Length–weight relations and chemical content of the planktonic copepods in the Cananeia lagoon estuarine system, Sao Paulo, Brazil. Plankton Biol Ecol 48:35–45
Baines SB, Pace ML, Karl DM (1994) Why does the relationship between sinking flux and planktonic primary production differ between lakes and oceans? Limnol Oceanogr 39:213–226
Banse K (1995) Zooplankton: pivotal role in the control of ocean production. ICES J Mar Sci 52:265–277
Blanchot J, André JM, Navarette C, Neveux J, Radenac M-H (2001) Picophytoplankton in the equatorial Pacific: vertical distributions in the warm pool and in the high nutrient low chlorophyll conditions. Deep Sea Res I 48:297–314
Bollens G, Gifford SM, Slaughter AM, Bollens SM (2005) Microzooplankton in the northern San Francisco estuary: important food resources but minimal phytoplankton grazers. ALSO, Salt Lake City
Bøsheim KY, Bratbak G (1987) Cell volume to cell carbon conversion factors for a bacterivorus Monas sp. enriched from sea waters. Mar Ecol Prog Ser 36:171–175
Bouley P, Kimmerer WJ (2006) Ecology of a highly abundant, introduced cyclopoid copepod in a temperate estuary. Mar Ecol Prog Ser 324:219–228
Calbet A, Landry MR (1999) Mesozooplankton influences on the microbial food web: direct and indirect trophic interactions in the oligotrophic open ocean. Limnol Oceanogr 44:1370–1380
Calbet A, Trepat I, Almeda R, Salo V, Saiz E, Movilla JI, Alcaraz M, Yebra L, Simo R (2008) Impact of micro-and nanograzers on phytoplankton assessed by standard and size-fractionated dilution grazing experiments. Aquat Microb Ecol 50:145–156
Caron DA, Dam HG, Kremer P, Lessard EJ, Madin LP, Malone TC, Napp JM, Peele ER, Roman MR, Youngbluth MJ (1995) The contribution of microorganisms to particulate carbon and nitrogen in surface waters of the Sargasso Sea near Bermuda. Deep-Sea Res I 42:943–972
Chen B, Liu H (2010) Relationships between phytoplankton growth and cell size in surface oceans: interactive effects of temperature, nutrients, and grazing. Limnol Oceanogr 55:965–972
Chen Y-LL, Chen H-Y, Gong G-C, Lin Y-H, Jan S, Takahashi M (2004) Phytoplankton production during a summer coastal upwelling in the East China Sea. Cont Shelf Res 24:1321–1338
Cho BC, Azam F (1990) Biogeochemical significance of bacterial biomass in the ocean’s euphotic zone. Mar Ecol Prog Ser 63:253–259
Christian JR, Karl DM (1994) Microbial community structure at the U.S.-Joint Global Ocean Flux Study Station ALOHA: inverse methods for estimating biochemical indicator ratios. J Geophys Res 99:14269–14276
Chuang W-S, Li H-W, Tang T, Wu C-K (1993) Observations of the countercurrent on the inshore side of the Kuroshio northeast of Taiwan. J Oceanogr 49:581–592
Edler L (1979) Phytoplankton and chlorophyll recommendations for biological studies in the Baltic Sea: phytoplankton and chlorophyll. Baltic Mar Biol Publ 5:1–38
Gasol J, Del Giorgio P, Duarte C (1997) Biomass distribution in marine planktonic communities. Limnol Oceanogr 42:1353–1363
Gifford D (1985) Laboratory culture of marine planktonic oligotrichs (ciliophora, oligotrichida). Mar Ecol Prog Ser 23:257–267
Gong G-C, Lee Chen Y-L, Liu K–K (1996) Chemical hydrography and chlorophyll a distribution in the East China Sea in summer: implications in nutrient dynamics. Cont Shelf Res 16:1561–1590
Gundersen K, Heldal M, Norland S, Purdie DA, Knap AH (2002) Elemental C, N, and P Cell Content of Individual Bacteria Collected at the Bermuda Atlantic Time-Series Study (BATS) Site. Limnol Oceanogr 47:1525–1530
Haury LR (1988) Vertical distribution of Pleuromamma (copepoda: Metridinidae) across the eastern north Pacific Ocean. Hydrobiologia 167(168):335–342
Huo Y-Z, Wang S-W, Sun S, Li C-L, Liu M-T (2008) Feeding and egg production of the planktonic copepod Calanus sinicus in spring and autumn in the Yellow Sea, China. J Plankton Res 30:723–734
Ishizaka J, Kiyosawa H, Ishida K, Ishikawa K, Takahashi M (1994) Meridional distribution and carbon biomass of autotrophic picoplankton in the central north Pacific Ocean during late northern summer 1990. Deep-Sea Res I 41:1745–1766
Iversen M, Poulsen L (2007) Coprorhexy, coprophagy, and coprochaly in the copepods Calanus helgolandicus, Pseudocalanus elongatus, and Oithona similis. Mar Ecol Prog Ser 350:79–89
Jang M-C, Shin K, Lee T, Noh I (2010) Feeding selectivity of calanoid copepods on phytoplankton in Jangmok Bay, south coast of Korea. J Ocean Sci 45:101–111
Jens CN, Lars-Johan N, Andrey S (2001) Correcting for underestimation of microzooplankton grazing in bottle incubation experiments with mesozooplankton. Mar Ecol Prog Ser 221:59–75
Jochem FJ (2003) Photo- and heterotrophic pico- and nanoplankton in the Mississippi River plume: distribution and grazing activity. J Plankton Res 25:1201–1214
Johnson PW, Sieburth JM, Xu H-S (1979) The utilization of crococcoid cyanobacteria by marine protozooplankters but not by calanoid copepods. Ann Inst Oceanogr 58:297–305
Jonsson PR, Tiselius P (1990) Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar Ecol Prog Ser 60:35–44
Kawasaki N, Sohrin R, Ogawa H, Nagata T, Benner R (2011) Bacterial carbon content and the living and detrital bacterial contributions to suspended particulate organic carbon in the North Pacific Ocean. Aquat Microb Ecol 62:165–176
Klein Breteler WCM, Schogt N, Baas M, Schouten S, Kraay GW (1999) Trophic upgrading of food quality by protozoans enhancing copepod growth: role of essential lipids. Mar Biol 135:191–198
Ko AR, Ju S-J, Lee C-R (2009) The physiological and ecological comparisons between warm (Pleuromamma sp.) and cold water copepod species (Neocalanus plumchrus) in the northwestern Pacific Ocean using lipid contents and compositions. Ocean Polar Res 31:121–131
Landry MR, Calbet A (2004) Microzooplankton production in the oceans. ICES J Mar Sci 61:501–507
Lee S, Fuhrman JA (1987) Relationship between biovolume and biomass of naturally derived marine bacterioplakton. Appl Environ Microbiol 53:1298–1303
Mackey DJ, Parslow J, Higgins HW, Griffiths FB, O’Sullivan JE (1995) Plankton productivity and biomass in the western equatorial Pacific: biological and physical controls. Deep-Sea Res II 42:499–533
Matsuno K, Yamaguchi A (2010) Abundance and biomass of mesozooplankton along north-south transects (165°E and 165°W) in summer in the North Pacific: an analysis with an optical plankton counter. Plankton Benthos Res 5:123–130
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Nakamura Y, Turner JT (1997) Predation and respiration by the small cyclopoid copepod Oithona similis: how important is feeding on ciliates and heterotrophic flagellates? J Plankton Res 19:1275–1288
Nejstgaard JC, Gismervik I, Solberg PT (1997) Feeding and reproduction by Calanus finmarchicus, and microzooplankton grazing during mesocosm blooms of diatoms and the coccolithophore Emiliania huxleyi. Mar Ecol Prog Ser 147:197–217
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon, New York
Pérez V, Fernández E, Marañón E, Serret P, Varela R, Bode A, Varela M, Varela MM, Morán XAG, Woodward EMS, Kitidis V, García-Soto C (2005) Latitudinal distribution of microbial plankton abundance, production, and respiration in the Equatorial Atlantic in autumn 2000. Deep-Sea Res I 52:861–880
Poulsen LK, Kiørboe T (2006) Vertical flux and degradation rates of copepod fecal pellets in a zooplankton community dominated by small copepods. Mar Ecol Prog Ser 323:195–204
Putt M, Stoecker DK (1989) An experimentally determined carbon: volume ratio for marine ‘‘oligotrichous’’ ciliates from estuarine and coastal waters. Limnol Oceanogr 34:1097–1103
R Development Team (2006) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.Rproject.Org/
Rassoulzadegan F, Laval-Peuto M, Sheldon RW (1988) Partitioning of the food ration of marine ciliates between pico- and nanoplankton. Hydrobiologia 159:75–88
Roman MR, Gauzens AL (1997) Copepod grazing in the equatorial Pacific. Limnol Oceanogr 42:623–634
Roman MR, Dam HG, Gauzens AL, Urban-Rich J, Foley DG, Dickey TD (1995) Zooplankton variability on the equator at 140°W during the JGOFS EqPac study. Deep-Sea Res II 42:673–693
Roman MR, Dam HG, Le Borgne R, Zhang X (2002) Latitudinal comparisons of equatorial Pacific zooplankton. Deep-Sea Res II 49:2695–2711
Saiz E, Calbet A (2011) Copepod feeding in the ocean: scaling patterns, composition of their diet and the bias of estimates due to microzooplankton grazing during incubations. Hydrobiologia 666:181–196
Samuelsson K, Berglund J, Andersson A (2006) Factors structuring the heterotrophic flagellate and ciliate community along a brackish water primary production gradient. J Plankton Res 28:345–359
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Antonie Leewenhoek Int J Gen Mol Microbiol 81:293–308
Sherr EB, Sherr BF (2007) Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar Ecol Prog Ser 352:187–197
Sherr EB, Sherr BF, Paffenhofer G (1986) Phagotrophic protozoa as food for metazoans: a “missing link” in marine pelagic food webs? Mar Microb Food Webs 1:61–80
Sherr EB, Caron DA, Sherr EB (1993) Staining of heterotrophic protists for visualization via epifluorescence microscopy. In: Kemp PF, Sherr EB, Cole JJ (eds) Hand book of methods in aquatic microbial ecology. Lewis, Boca Raton, pp 213–227
Stoecker DK, Capuzzo JM (1990) Predation on protozoa: its importance to zooplankton. J Plankton Res 12:891–908
Stoecker DK, Gifford DJ, Putt M (1994) Preservation of marine planktonic ciliates: losses and cell shrinkage during fixation. Mar Ecol Prog Ser 110:293–299
Strom SL, Macri EL, Olson NB (2007) Microzooplankton grazing in the coastal Gulf of Alaska: variations in top-down control of phytoplankton. Limnol Oceanogr 52:1480–1494
Takahashi M, Bienfang PK (1983) Size structure of phytoplankton biomass and photosynthesis in subtropical Hawaiian waters. Mar Biol 76:203–211
Tang KW, Taal M (2005) Trophic modification of food quality by heterotrophic protists: species-specific effects on copepod egg production and egg hatching. J Exp Mar Biol Ecol 31:85–98
Tirelli V, Mayzaud P (2005) Relationship between functional response and gut transit time in the calanoid copepod Acartia clausi: role of food quantity and quality. J Plankton Res 27:557–568
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud 43:255–266
Vargas CA, Gonzalez HE (2004) Plankton community structure and carbon cycling in a coastal upwelling system. II. Microheterotrophic pathway. Aquat Microb Ecol 34:165–180
Venables WN, Ripley BR (2003) Modern applied statistics with S, 4th edition. Springer, New York
Verity P (1985) Grazing, respiration, excretion, and growth rates of tintinnids. Limnol Oceanogr 30:1268–1282
Verity PG, Lagdon C (1984) Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. J Plankton Res 6:859–868
Wassmann P (1990) Relationship between primary and export production in the boreal coastal zone of the North Atlantic. Limnol Oceanogr 35:464–471
Yamaguchi A, Watanabe Y, Ishida H, Harimoto T, Furusawa K, Suzuki S, Ishizaka J, Ikeda T, Takahashi MM (2004) Latitudinal differences in the planktonic biomass and community structure down to the greater depths in the Western North Pacific. J Oceanogr 60:773–787
Yamaguchi A, Watanabe Y, Ishida H, Harimoto T, Maeda M, Ishizaka J, Ikeda T, Mac Takahashi M (2005) Biomass and chemical composition of net-plankton down to greater depths (0–5800 m) in the western North Pacific Ocean. Deep-Sea Res I 52:341–353
Yang EJ, Choi JK, Hyun JH (2008a) Seasonal variation in the community and size structure of nano-and microzooplankton in Gyeonggi Bay, Yellow Sea. Estuar Coast Shelf Sci 77:320–330
Yang EJ, Ju S-J, Kim WS (2008b) Regional comparisons of heterotrophic protists grazing impacts and community in Northwest Pacific Ocean. Ocean Polar Res 30:289–301
Yang EJ, Kang H-K, Yoo S, Hyun J-H (2009) Contribution of auto- and heterotrophic protozoa to the diet of copepods in the Ulleung Basin, East Sea/Japan Sea. J Plankton Res 31:647–659
Zarauz I, Irigoien X (2008) Effects of Lugol’s fixation on the size structure of natural nano-microplankton samples, analyzed by means of an automatic counting method. J Plankton Res 30:1297–1303
Zhang J, Liu SM, Ren JL, Wu Y, Zhang GL (2007) Nutrient gradients from the eutrophic Changjiang (Yangtze River) Estuary to the oligotrophic Kuroshio waters and re-evaluation of budgets for the East China Sea Shelf. Prog Oceanogr 74:449–478
Acknowledgments
We are indebted to the captain and crew of the R/V Eardo, who were most helpful with all our shipboard operations. This work was supported by the KORDI project PE98731 and PM56600. E.J. Yang was supported by KOPRI project (PE10290).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CR., Choi, KH., Kang, HK. et al. Biomass and trophic structure of the plankton community in subtropical and temperate waters of the northwestern Pacific Ocean. J Oceanogr 68, 473–482 (2012). https://doi.org/10.1007/s10872-012-0111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-012-0111-2