Abstract
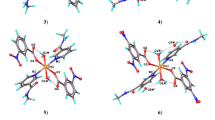
The compound has been formed by mononuclear [Cu(C9H7O4)2(C6H6N2O)2(H2O)] units in which the metal ion as well as the water ligand lies on a twofold symmetry axis, so that only one acetylsalicylate ligand and one nicotinamide ligand are independent. The distortion from ideal five-coordinate geometries can be described best by the degree of trigonality τ. For a regular square-pyramidal (SQP) geometry the trigonality parameter is 0 and for a trigonal–bipyramidal (TBP) structure it increases to 1. The copper coordination geometry is that of a square pyramid (τ = 0.23), with the N atoms from nicotinamide ligands and the bonded carboxylate O atoms from acetylsalicylate ligands defining the quasi-planar square base. The apical site is occupied by the aqua ligand, a bond which coincides with the twofold symmetry axis and is thus exactly perpendicular to the basal plane. The thermal decomposition takes place in four steps: removing of moisture, dehydration of aqua ligand, the elimination of the nicotinamide (na) ligand and the decomposition of acetyl-groups and oxidation of salicylate ion ligands. In complex, all ligands are coordinated to the metal ion as monodendate. The IR spectra of the intermediate products showed similar results.
Graphical Abstract
The compound is formed by mononuclear [Cu(C9H7O4)2(C6H6N2O)2(H2O)] units, in which the metal ion as well as the water ligand lie on a twofold symmetry axis, so that only one acetylsalicylate ligand and one nicotinamide ligand are independent. The framework of compound is put in order as layers. The distortion from ideal five-coordinate geometries can be best described by the degree of trigonality τ.












Similar content being viewed by others
References
Yeşilel OZ, Olmez H, Uçar I, Bulut A, Kazak C (2005) Z Anorg Allg Chem 631:3100
Chenoweth MB (1956) Pharmocol Rev 8:57
Sorenson JRJ (1982) In: Siegel H (ed) Metal Ions in biologicals, vol 14. Marcel-Dekker, New York
Reid J, Watson RD, Cochran JB, Sproull DH (1951) Br Med J 1:321
Olmez H, Arslan F, Icbudak H (2004) J Therm Anal Cal 76:793
Geraghty M, Sheridan V, McCann M, Devereux M, McKee V (1999) Polyhedron 18:2931
Icbudak H, Heren Z, Kose DA, Necefoglu H (2004) J Therm Anal Cal 76:837
Kose DA, Necefoğlu H (2008) J Therm Anal Cal 93(2):509
Kose DA, Zumreoglu-Karan B, Unaleroglu C, Şahin O, Buyukgungor O (2006) J Coord Chem 17:2125
Kose DA, Kaya A, Necefoglu H (2007) Russ J Coord Chem 33(6):422
Kose DA, Içbudak H, Necefoglu H (2007) Hacettepe J Biol Chem 35(2):123
Wolodkiewicz W, Brzyska W (1997) Polish J Chem 71:16
Erdelyiová A, Györyová K, Gyepes R, Halás L, Kováŕová J (2009) Polyhedron 28:131
Kose DA, Necefoglu H, Icbudak H (2008) J Coord Chem 61(21):3508
Kose DA, Gokce G, Gokce S, Uzun I (2009) J Therm Anal Cal 95(1):247
Icbudak H, Olmez H, Yesilel OZ, Arslan F, Naumov P, Jovanovski G, Ibrahim AR, Usman A, Fun HK, Chantrapromma S, Ng SW (2003) J Mol Struct 55:6572
Sutton D (1968) Electronic spectra of transition metal complexes. McGraw-Hill, London
Zeleñák V, Vargová Z, Györyová K (2007) Spectrochim Acta A 66:262
Lee JD (2002) Concice inorganic chemistry, 5th edn. Blackweel Science, London
Içbudak H, Yazicilar TK, Yilmaz VT (1999) Thermochim Acta 335:93
Kose DA, Ozturkkan EF, Necefoglu H, Uzun I (2007) Assian J Chem 19(6):4880
Içbudak H, Yilmaz VT, Olmez H (1998) J Therm Anal 53:843
Sheldrick GM (2008) Acta Cryst A64:112
Farrugia LJ (1999) J Appl Cryst 32:837
Spek AL (2003) J Appl Cryst 36:7
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555
Addison AW, Rao TN, Reedijk J, Rijn JV, Verschoor GC (1984) J Chem Soc Dalton Trans 1349
Brown ID (1994) In: Bürgi HB, Dunitz JD (eds) Bond-length-bond-valence relationship in inorganic solids, structure correlation, part III. VCH Publishers, Weinheim/New York, pp 405–429
O’Keeffe M, Brese NE (1991) J Am Chem Soc 113:3226
Brown ID (2002) The chemical bond in inorganic chemistry. The bond valence model. Oxford University Press, Oxford
Shields GP, Raithby PR, Allen FH, Motherwell WDS (2000) Acta Cryst B56:455
Flack HD (1983) Acta Cryst A39:876
Acknowledgments
The authors wish to acknowledge the Faculty of Arts and Sciences, Ondokuz Mayıs University, Turkey, for the use of the Stoe IPDS-II diffractometer (purchased from Grant No. F279 of the University Research Fund).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kose, D.A., Necefoğlu, H., Şahin, O. et al. Synthesis, Spectral, Thermal and Structural Study of Monoaquabis(Acetylsalicylato-κO)bis(Nicotinamide-κN)Copper(II). J Chem Crystallogr 41, 297–305 (2011). https://doi.org/10.1007/s10870-010-9876-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9876-6