Abstract
The synthesis of six new copper(II) nitrobenzoate complexes with N-methylnicotinamide, used as an auxiliary ligand for a supramolecular interaction study, is reported. Crystal structures of six novel compounds [Cu(2-NO2bz)2(mna)2(H2O)2] (1), [Cu(2-NO2bz)2(mna)2(H2O)2]∙2H2O (2), [Cu(3-NO2bz)2(mna)2(H2O)2] (3), [Cu(3,5-(NO2)2bz)2(mna)2(H2O)2]∙2H2O (4), [Cu(4-NO2bz)2(mna)2(H2O)2]∙2(4-NO2bzH) (5) and [Cu(3,5-(NO2)2bz)2(mna)(H2O)3] (6) (mna = N-methylnicotinamide, 2-NO2bz = 2-nitrobenzoate, 3-NO2bz = 3-nitrobenzoate, 4-NO2bz = 4-nitrobenzoate, 3,5-(NO2)2bz = 3,5-dinitrobenzoate) were determined by X-ray analysis. Compounds 1–6 are mononuclear with a tetragonal-bipyramidal geometry around the Cu2+ ion. The molecules of the studied complexes are mostly linked by a combination of N–H…O and O–H…O hydrogen bonds between N-methylnicotinamide and water molecules into supramolecular hydrogen-bonded coordination chains and networks. Intermolecular interactions in the supramolecular structures were also studied using Hirshfeld surface analysis. In addition, the complexes 1–6 have been characterised by elemental analysis, IR, UV–Vis and EPR spectroscopy. Density functional theory calculations were performed in order to reproduce the EPR magnetic parameters. DFT calculations of the EPR parameters show a good agreement with the experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The field of structural and supramolecular chemistry has developed rapidly in the study of metal–organic supramolecular systems, partly because of the intellectual challenge of controlling the self-assembly processes and partly because of the potential applications of such novel materials in magnetic devices, nonlinear optical materials, heterogeneous catalysis, microporous and zeolite-like materials [1,2,3,4,5]. The formation of various metal–organic supramolecular frameworks is caused by a spontaneous self-assembly process of metal cations and ligands via metal–ligand coordination in combination with other noncovalent interactions such as hydrogen bonding, π–π interactions or dispersive interactions [6].
Excellent examples of ligands for the construction of supramolecular architectures in the metal complexes are pyridincarboxamides such as nicotinamide and its derivatives [7]. Nicotinamide is one of the most extensively studied pyridine derivatives. It is a component of the vitamin B complex and also forms part of the vital coenzyme NAD (nicotinamide adenine dinucleotide) [8]. It is also a typical N-donor ligand, widely used in crystal engineering and supramolecular chemistry [9,10,11,12,13]. Moreover, copper(II) carboxylate complexes with nicotinamide or isonicotinamide could have a potential utilization in supramolecular medicinal chemistry [14, 15]. Nicotinamide as a ligand in the transition metal complexes creates the hydrogen-bonded coordination networks through the carboxamide group [7, 9]. The hydrogen-bonded metal–organic supramolecular systems based on nicotinamide have been extensively studied in other papers [16,17,18,19,20,21,22,23]. On the other hand, only a few papers report on transition metal complexes with N-methylnicotinamide as a hydrogen-bonded linking agent for the construction of different supramolecular architectures [24,25,26,27,28,29,30].
In this respect, we report a synthesis, structural, spectral and DFT characterization of six new monomeric copper(II) nitrobenzoate complexes with N-methylnicotinamide: [Cu(2-NO2bz)2(mna)2(H2O)2] (1), [Cu(2-NO2bz)2(mna)2(H2O)2]∙2H2O (2), [Cu(3-NO2bz)2(mna)2(H2O)2] (3), [Cu(3,5-(NO2)2bz)2(mna)2(H2O)2]∙2H2O (4), [Cu(4-NO2bz)2(mna)2(H2O)2]∙2(4-NO2bzH) (5) and [Cu(3,5-(NO2)2bz)2(mna)(H2O)3] (6) (mna = N-methylnicotinamide, 2-NO2bz = 2-nitrobenzoate, 3-NO2bz = 3-nitrobenzoate, 4-NO2bz = 4-nitrobenzoate, 3,5-(NO2)2bz = 3,5-dinitrobenzoate). The crystal packing and intermolecular noncovalent interactions in the complexes have been further studied by means of Hirshfeld surface analysis.
Experimental
Preparation of complexes
[Cu(2-NO2bz)2(mna)2(H2O)2] (1)
The complex 1 was prepared by the reaction of an acetonitrile solution (100 ml) of copper(II) acetate (0.5 mmol, 0.100 g) with N-methylnicotinamide (1 mmol, 0.136 g) followed by addition of the 2-nitrobenzoic acid (1 mmol, 0.167 g). After a few hours of stirring, the resulting turquoise solution was left to crystalize at ambient temperature. The blue crystals suitable for X-ray structure determination were separated after a several weeks. Yield: 0.34 g (49%). Anal. calc. for C28H28O12N6Cu (Mr = 704.11): C 47.76, H 4.01, N 11.94. Found: C 48.02, H 4.22, N 12.05%. IR (ATR, cm−1): 3501 (m), 3326 (m), 3065 (w), 1662 (m), 1600 (m), 1591 (m), 1560 (m, sh), 1529 (vs), 1376 (vs), 1298 (s), 1195 (m), 1156 (w), 1033 (m), 956 (m), 823 (m), 769 (m), 697 (vs), 651 (m), 576 (m), 432 (m). UV–VIS (nm): 267, 325 (sh) 631(br).
[Cu(2-NO2bz)2(mna)2(H2O)2]∙2H2O (2)
The complex 2 was prepared as described above for 1, except that the synthesis was carried out in aqueous solution. Yield: 0.15 g (41%). Anal. calc. for C28H32O14N6Cu (Mr = 740.15): C 45.44, H 4.36, N 11.36. Found: C 45.49, H 4.14, N 11.46. IR (ATR, cm−1): 3381 (m, sh), 3305 (m), 3240 (m, sh), 3089 (w), 1650 (m), 1605 (m), 1590 (m), 1572 (s), 1524 (vs), 1480 (m), 1384 (s), 1346 (vs), 1200 (m), 1178 (m), 1151 (m), 1061 (m), 1034 (m), 945 (w), 833 (m), 784 (m), 748 (m), 695 (s), 649 (m), 457 (m), 432 (m). UV–VIS (nm): 268, 321 (sh), 638 (br).
[Cu(3-NO2bz)2(mna)2(H2O)2] (3)
The complex 3 was prepared by the reaction of an aqueous solution (100 ml) of copper(II) acetate (0.5 mmol, 0.100 g) with N-methylnicotinamide (1 mmol, 0.136 g) followed by addition of the 3-nitrobenzoic acid (1 mmol, 0.167 g). The reaction mixture was stirred until the reaction finished and the color of the product remained unchanged. The blue microcrystals were filtered off, washed with small portion of water and dried at ambient temperature. The resulting solution obtained from filtration was kept for evaporation at ambient temperature. The blue crystals suitable for X-ray structure determination were separated after a several days. Yield: 0.20 g (57%). Anal. calc. for C28H28O12N6Cu (Mr = 704.11): C 47.76, H 4.01, N 11.94. Found: C 47.39, H 4.25, N 11.80%. IR (ATR, cm−1): 3471 (m), 3328 (m), 3089 (w), 1666 (m), 1602 (m), 1593 (m), 1545 (s), 1527 (vs), 1475 (m), 1388 (s), 1348 (vs), 1307 (m), 1268 (m), 1191 (m), 1072 (m), 843 (m), 791 (m), 715 (s), 698 (s), 652 (m), 592 (w), 541 (w), 439 (m). UV–VIS (nm): 267, 331 (sh), 618 (br).
[Cu(3,5-(NO2)2bz)2(mna)2(H2O)2]∙2H2O (4)
The complex 4 was prepared in similar manner as 3 except that 3,5-dinitrobenzoic acid (1 mmol, 0.212 g) was used. Yield: 0.28 g (68%). Anal. calc. for C28H30O18N8Cu (Mr = 830.15): C 40.51, H 3.64, N 13.50%. Found: C 40.21, H 3.36, N 13.43. IR (ATR, cm−1): 3492 (m), 3361 (m, sh), 3247 (m, br), 3075 (m), 1651 (m), 1621 (s), 1604 (m), 1578 (m), 1533 (vs), 1458 (m), 1388 (m), 1340 (vs), 1197 (m), 1090 (m), 1062 (m), 927 (m),918 (m), 833 (m), 793 (m), 721 (vs), 700 (s), 651 (m), 521 (m), 435 (m). UV–VIS (nm): 262, 318 (sh), 643 (br).
[Cu(4-NO2bz)2(mna)2(H2O)2]∙2(4-NO2bzH) (5)
The complex 5 was prepared as described for 3 except that 4-nitrobenzoic acid (2 mmol, 0.234 g) was used and the molar ratio copper(II) acetate: N-methylnicotinamide has been changed from 1: 2 to 1: 1 (1 mmol, 0.136 g of N-methylnicotinamide). Yield: 0.45 g (43%). Anal. calc. for C42H38O20N8Cu (Mr = 1038.34): C 48.68, H 3.69, N 10.79. Found: C 48.49, H 3.46, N 10.71%. IR (ATR, cm−1): 3545 (m), 3368 (m), 3107 (w), 2940 (m, sh), 2622 (w), 1707 (s), 1645 (m), 1615 (m), 1585 (s), 1515 (s), 1406 (m), 1375 (m), 1344 (s), 1317 (vs), 1242 (s), 1195 (m), 1105 (m), 1057 (m), 1011 (m), 893 (m), 871 (s), 784 (m), 717 (s), 701 (vs), 647 (m), 502 (m), 434 (m). UV–VIS (nm): 274, 315 (sh), 623 (br).
[Cu(3,5-(NO2)2bz)2(mna)(H2O)3] (6)
The complex 6 was prepared as described for 5 except that 3,5-dinitrobenzoic acid (2 mmol, 0.424 g) was used. Yield: 0.57 g (84%). Anal. calc. for C21H20O16N6Cu (Mr = 675.97): C 37.31, H 2.98, N 12.43. Found: C 37.16, H 3.04, N 12.82%. IR (ATR, cm−1): 3535 (m), 3443 (m), 3233 (m, br), 3100 (m), 1622 (s), 1575 (m), 1537 (s), 1455 (m), 1414 (m), 1374 (m, sh), 1340 (vs), 1327 (s, sh), 1182 (m), 1070 (m), 909 (m), 792 (m), 719 (vs), 647 (m), 557 (m), 521 (m), 438 (m). UV–VIS (nm): 265, 320 (sh), 690 (br).
Physical measurements
Carbon, hydrogen and nitrogen analyses were carried out on a CHNSO FlashEA™ 1112 Automatic Elemental Analyzer. The electronic spectra (190–1100 nm) of the complexes in the solid state were measured in nujol suspension with a SPECORD 250 Plus (Carl Zeiss Jena) spectrophotometer at room temperature. The infrared solid state spectra (ATR technique, 4000–400 cm−1) were recorded on a Nicolet 5700 FT-IR spectrophotometers at room temperature. Room-temperature EPR spectra of the powdered samples were recorded on an EMX Plus series EPR spectrometer (Bruker, Germany) operating at X-band (≈ 9.4 GHz) and simulated using Spin.exe software developed by Dr. Ozarowski [31].
X-ray crystallography
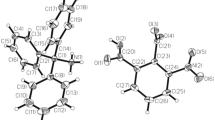
Data collection and cell refinement for complexes under study were measured on a Bruker-Nonius Kappa CCD diffractometer with an APEX-II CCD detector using graphite monochromated Mo-Kα radiation. Absorption correction was applied by multi-scan method using SADABS program [32]. The structures were solved by a direct method with ShelXS-2013 [33] or SIR-2011 [34] and refined by the full-matrix least squares procedure with either ShelXL [35] or Olex2.refine [36]. Geometrical analysis was performed using ShelXL and the structures were drawn using the OLEX2 (ver. 1.5) [37] or MERCURY (ver. 3.8) [38] programs. ORTEP-like representations of the molecular structure of all complexes with descriptions of all non-hydrogen atoms are presented in the supplementary material as Figs. S1–S6. Table 1 shows the final crystal data and structure refinement parameters.
Nitro groups in crystal structures: 3 (nitro group of the 3-nitrobenzoate ligand), 4 (two nitro groups of the 3,5-dinitrobenzoate ligand) and 6 (two nitro groups of a 3,5-dinitrobenzoate ligand) are disordered in two positions with occupation factors 0.25/0.75; 0.15/0.85; 0.77/0.23; 0.77/0.23; 0.20/0.80, respectively. Hydrogen atoms of water molecules were found from the difference map or added and refined using a utility in OLEX2. They were then fixed at the refined ideal distances. Hydrogen atoms of other groups were added at standard distances.
Hirshfeld surface analyses
CrystalExplorer [39] was used to calculate Hirshfeld surfaces [40, 41] and associated fingerprint plots [42, 43]. The Hirshfeld surfaces have been calculated including all orientations of the disordered methyl groups with their partial occupancies. Hirshfeld surfaces for disordered structures (3, 4 and 6) have been calculated for both disordered parts.
DFT calculations and computational details
The theoretical DFT calculation of g factors and A tensors were done with ORCA (version 4.0.0.2) program package [44] using methods developed and implemented into ORCA by Neese [45, 46]. Geometry optimization was done with the BP86 functional with Grimme dispersion correction method D3BJ on the experimental geometries obtained directly from the X-ray analysis. Tight convergence criteria and increased integration grids (final grid 5) were applied throughout. The def2-TZVP basis set together with corresponding auxilliary basis set was employed in the calculation. Two different functional have been used in the calculation: one hybrid functional, B3LYP and one hybrid meta-GGA functional, TPSSh. Relativistic effects were included using the Douglas-Kroll-Hess transformation to second order. The aug-cc-pVTZ-J basis set was employed for Cu [47] in the computation of spin hamiltonian parameters using enhanced grid for copper (SpecialGridIntAcc 7). All other atoms utilized relativistically recontracted Ahlrich’s dkh-def2-TZVP basis set [48, 49]. Spin–orbit mean field approach SOMF(1X) with one-center approximation to the exchange term were also applied. To speed up the calculation the chain-of-spheres approximation (RIJCOSX) was set on using an automatically generated auxiliary basis set [50]. In order to increase convergence tolerances and integration accuracy, TightSCF and Grid5 options were used. All our calculation of molecular g and A tensors have been done in the gas phase and we have made no attempt to simulate environmental effects on the computed values.
Results and discussion
Synthesis
The reaction between copper acetate dihydrate, N-methylnicotinamide and the corresponding nitrobenzoic acid in appropriate molar ratios in acetonitrile (1) or aqueous solution (2–6) leads to the formation of complexes of the general formulae: [Cu(X-NO2bz)2(mna)2(H2O)2] (1–3, 5) (where X = 2, 3 or 4) or [Cu(3,5-NO2bz)2(mna)2(H2O)2] (4) or [Cu(3,5-NO2bz)2(mna)(H2O)3] (6). A schematic representation of the preparation procedure is summarised in Scheme 1. All six complexes were isolated in a good yields (41–84%) and are stable in the air. The composition of the complexes was verified by the results of elemental analysis and IR spectroscopy and finally fully confirmed by single crystal X-ray diffraction analysis.
FT-IR and electronic spectroscopy
The IR spectra of the complexes 1–6 were measured in the range of 4000–400 cm−1 in the solid state by ATR technique (Figs. S7–S12) and a tentative band assignment of the main functional groups of the bound ligands (water, nitrobenzoates and N-methylnicotinamide) was done based on the literature [51]. In general, the observed main features of the IR spectra were clearly consistent with the composition and structural features of the prepared complexes. Some selected IR bands of complexes 1–6 are collected in Table 2. The IR spectra of all studied complexes showed broad peaks of medium intensity in the region 3500–3100 cm−1, which can be attributed to O–H (broader peaks at 3501–3444 cm−1) and N–H (sharper peaks at 3368–3305 cm−1) stretching vibrations of coordinated water and N-methylnicotinamide. Moreover, the complexes containing a lattice water molecules (2,4) or an additional water molecule (6) in addition to the coordinated axial water molecules also showed another broad band in this region (Figs. S8, S10, S12). The weak absorption bands observed in the region 3100–2600 cm−1 were attributed to C–H stretching vibrations of the coordinated nitrobenzoate and N-methylnicotinamide ligands. The carboxylate coordination mode of nitrobenzoates can be verified by the observation of antisymmetric and symmetric stretching vibrations of the carboxyl group, found in the region 1585–1544 cm−1 and 1388–1374 cm−1 for the studied complexes. The parameter ∆ (ῦa(COO)–ῦs(COO)) which is used in this context shows values from 184 cm−1 for 1 to 210 for 5 cm−1, except for complex 3 (∆ = 157 cm−1). These values indicate a monodentate type of coordination of the nitrobenzoate ligand, in agreement with literature data [19, 52]. The lower observed value of the ∆ parameter for complex 3 does not imply a different coordination mode of the 3-nitrobenzoate ligand, but can be explained by the presence of a combination band (mixed asymmetric NO2 and COO− vibration) in the IR spectrum, which hinders the more accurate wavenumber determination of ῦa(COO). Furthermore, the IR spectrum of 5 contains bands at 1707 and 1242 cm−1 respectively, which can be attributed to C=O and C–O stretching frequencies of 4-nitrobenzoic acid, which is bound by strong hydrogen bonds in the second coordination sphere of the complex. The most intense absorption bands in the IR spectra of all complexes were bands found between 1544–1515 cm−1 and 1376–1317 cm−1 which can be assigned to asymmetric and symmetric stretching vibrations of the nitro group [53].
The most characteristic absorption bands of secondary amides, such as N-methylnicotinamide, are the stretching vibration bands attributed to the N–H vibration and the mixed vibration called amide I, corresponding mainly to the C=O vibration, which could be easily identified in the IR spectra [26]. These bands are found at 3329 cm−1 for N–H and 1640 cm−1 for amide I in the IR spectrum of solid N-methylnicotinamide. Both bands are strongly involved in the hydrogen bonding, as shown by the crystal structure of the solid ligand [26]. In this respect, the involvement of the amide group in the hydrogen bonding in the studied complexes can be followed from the position of the amide I band. The carbonyl group in amides acts as an acceptor of hydrogen in hydrogen bonding. In complexes 1 and 3, the carbonyl group is not involved in the hydrogen bonding (Table 4), hence the observed position of the amide I band (1662 and 1666 cm−1) is found at a higher wavenumber compared to the solid N-methylnicotinamide ligand (1640 cm−1). However, for complexes 5 and 6 with the strongest involvement of the carbonyl group in hydrogen bonding, the position of the amide I band is found at 1645 and 1622 cm−1, respectively. A very similar conclusion can be drawn for the position and shape of the N–H band (Table 2). Finally, the IR spectra of all complexes showed C=N and C=C vibrations within the aromatic moiety of the pyridine and benzene rings in the relatively narrow region 1590–1622 cm−1. Since these vibrations are relatively insensitive to the coordination to the metal centre, it is better to follow the shift of the bands corresponding to the in-plane pyridine ring deformation γ(CN) (619/633 cm−1 in N-methylnicotinamide), which are found in the range 647–652 cm−1 in our complexes, suggesting the coordination of the ligand to the copper atom through the pyridine nitrogen atom [51].
The solid state UV–Vis spectra of the studied monomeric complexes 1–6 exhibit a very similar pattern. The electronic spectra are shown in Fig. S13. As can be clearly seen (Fig. S13 right), these spectra show a broad asymmetric band with a maximum ranging from 618 nm (16,180 cm−1) for 3 to 690 nm (14,490 cm−1) for 6 in the visible region. This band corresponds to a spectroscopically forbidden electronic d-d transition from the predominantly dxy orbital to the dx2−y2 orbital (Fig. S13). Such a d-d transition is typical for complexes with the CuO4N2 (1–5) or CuO5N (6) chromophore with a tetragonally distorted octahedral environment around the copper(II) centre [54, 55]. Based on the position of the d-d peak in the spectrum, the complexes can be arranged as follows according to the observed tetragonal distortion around the copper atom: 3 (618 nm) > 5 (623 nm) > 1 (631 nm) > 2 (638 nm) > 4 (643 nm) > 6 (690 nm). The maximum of the d-d band in the case of complex 6 is found at the longest wavelength (690 nm) in this series of complexes, reflecting the presence of a lower ligand field around the copper ion as a result of a different chromophore (CuO5N) compared to the other complexes 1–5 (CuO4N2). In addition, the spectra also contain two bands at around 200–400 nm which could be considered as an intraligand transition (262–274 nm) and a ligand-to-metal charge transfer transition (shoulder at around 315–330 nm) between the π-electron cloud of an aromatic ligand moiety and a central copper atom [53, 55].
Molecular and crystal structures
The centrosymmetric molecules [Cu(X-NO2bz)2(mna)2(H2O)2] of compounds 1–5 are very similar. The molecular structures of complexes 1–5 are depicted in Fig. 1. The coordination environment around Cu(II) atoms in the 1–5 adopts tetragonal-bipyramidal (4+2) stereochemistry. The tetragonal plane in the complexes is formed by a pair of monodentately coordinated carboxylate anions with Cu–Oeq distances 1.95–1.97 Å and by a pair of neutral N-methylnicotinamide molecules that coordinates to the central atoms using pyridine ring nitrogen atom with Cu–Neq distances 2.02–2.04 Å in trans positions (Table 3).
Two water molecules with Cu–OWax distances 2.49–2.57 Å occupy axial positions. As a result of the presence of water molecules, the molecular structures of 1–5 are stabilised by intramolecular hydrogen bonds O1W–H1W···O2 (the distances O1W···O2 are in the range 2.66–2.74 Å) (Table 4). The molecular structure of the molecular complex [Cu(2-NO2bz)2(mna)2(H2O)2] in 1 and 2 is similar but differs in conformation and can therefore be considered as pseudopolymorphs. The angle between the plane of the nitro group and the plane of the carboxylate group of the 2-nitrobenzoate ligands in two pseudopolymorphs is 88.4° for 1 and 66.9° for 2 [56, 57].
The crystal structures of 1 and 3 are very similar, which simplifies the discussion of them. In the crystal structures of 1 and 3 (Fig. 2) are the molecules of [Cu(X-NO2bz)2(mna)2(H2O)2] (X = 2 or 3) arranged into the layers, which are connected through a system of N2–H2N···O1Wii hydrogen bonds [N2···O1Wii = 3.012(4) and 2.941(2) Å, respectively; symmetry code: (ii) − x + 1, − y + 2, − z + 1] between carboxamide groups of N-methylnicotinamide and coordinated water molecules; and through O1W–H2W···O2i hydrogen bonds [O1W···O2i = 2.961(4) and 2.901(2) Å, respectively; symmetry code: (i) − x, − y + 1, − z + 1] between coordinated water molecules and uncoordinated carboxyl oxygen atoms of carboxylate anions. The four O–H···O hydrogen bonds [O1W–H1W∙∙O2 and O1W–H2W···O2ii] create R42(8) supramolecular synthons (Fig. 2) [58]. On the other hand, the two complex molecules of 1 or 3, linked by N2–H2N···O1Wii hydrogen bonds, create R22(16) supramolecular synthons (Fig. 2) [58]. The crystal structures of zinc(II) complexes of the formula [Zn(X-sal)2(mna)2(H2O)2] (X-sal = 4-bromosalicylate, 4-chlorosalicylate or 5-chlorosalicylate) exhibit similar hydrogen bonding systems [59], except for the fact that the nitrogen carboxamide atoms are not involved in hydrogen bonding. The stronger hydrogen bonding system involving water molecules and carboxylato oxygen atoms gave the shortest Cu···Cu distances of 7.390(2) Å, and 7.307(3) Å for 1 and 3 respectively. The slightly weaker system with carboxamide groups resulted in the shortest Cu···Cu distances of 8.890(2) Å, and 8.805(3) Å for 1 and 3 respectively, forming a rhombohedral grid of copper(II) atoms within the layers. These layers are held together by weak van der Waals forces in a zipper-like manner with weak π-π stacking interactions [60] between two benzene rings of 2-nitrobenzoate ligands in 1 (Fig. S14) and 3-nitrobenzoate ligands in 3 (Fig. S15), that are significantly stronger in the case of 1. The centroid-to centroid distance cg···cg is 3.58 Å, and 4.16 Å for 1 and 3, respectively.
Symmetry codes: (i) − x, − y + 1, − z + 1; (ii) − x + 1, − y + 2, − z + 1; (iii) x, y + 1, z; (iv) − x + 2, − y + 1, − z + 1; (v) x + 1, y, z + 1; (vi) − x + 1, − y + 2, − z + 2; (vii) − x, − y + 1, − z + 2; (viii) x, − y + 1/2, z − 1/2; (ix) x, − y + 3/2, z − 1/2; (x) − x + 1, − y, − z + 1; (xi) x, y − 1, z.
The crystal structure of 1 and 3 also shows π-π stacking interactions [60] between two pyridine rings of N-methylnicotinamide ligands with a centroid-to centroid distance cg···cg of 3.94 Å (Fig. S16) and 3.84 Å (Fig. S17), respectively. The shortest interplanar Cu···Cu distances are 12.082(2) Å, and 12.957(4) Å for 1 and 2, respectively. The crystal structures of pseudopolymorphs 1 and 2 differ in that 2 contains uncoordinated lattice water molecules which are involved in hydrogen bonding. As a result, the hydrogen-bonding networks in the complex 2 (Fig. 2) are enriched. The same situation applies to complex 4 (Fig. 2). Squeezing the uncoordinated water molecules into hydrogen-bonding networks increases the number of O–H···O hydrogen bonds connected to the rings, but there is a big difference in the involvement of the water molecules. Both complexes 2 and 4 create R22(16) supramolecular synthons held by hydrogen bonding between coordinated water molecules and N-methylnicotinamide ligands (Fig. 2), but in two different ways. In complex 2, the supramolecular synthons R22(16) are formed between hydrogen atoms H2W of coordinated water molecules O1W and oxygen O2 acceptors of N-methylnicotinamide O1W–H2W···O2iii [O1W···O2iii = 2.740(6) Å, symmetry code: (iii) x, y + 1, z]. The R22(16) synthons in the complex 4 are similar to those in complexes 1 and 3, formed between hydrogen H2N atoms of carboxamide groups and water molecule oxygen O1W atoms N2–H2N···O1Wv in [N2···O1Wv = 2.902(7) Å, symmetry code: (v) x + 1, y, z + 1]. The uncoordinated water molecules influence the hydrogen bonding system of the coordinated water molecules and instead of the R42(8) synthons found in 1 and 3, there are six O–H···O hydrogen bonds in the structure of both complexes, that are involved in the formation of R64(12) supramolecular synthons [58]. But again there is a big difference between the two complexes and the connections of uncoordinated and coordinated water represent two alternatives of R64(12) ring formation. The R64(12) rings of 2 (Fig. 2) are created through O2W–H3W···O1W; O2W–H4W···O2iv and O1W–H1W···O2 [distances O···O of 2.795(6); 2.726(7) and 2.738(7) Å, respectively; symmetry code: (iv) − x + 2, − y + 1, − z + 1]. The R64(12) rings of 4 (Fig. 2) are formed through O1W–H2W···O2W; O2W–H4W···O2i and O1W–H1W···O2 [distances O···O of 2.730(7); 2.762(7) and 2.730(7) Å, respectively; symmetry code: (i) − x, − y + 1, − z + 1]. Consequently, the uncoordinated water molecules O2W and complex molecules of both complexes 2 and 4 are connected to R22(10) rings (Fig. 2) through two different alternatives of hydrogen bonds N2–H2N···O2W [N2···O2W = 2.838(6) Å] and O2W–H3W···O1W [O2W···O1W = 2.795(6) Å] in 2 or O1W–H2W···O2W [O1W···O2W = 2.739(7) Å] and O2W–H3W···O3 [O2W···O3 = 2.719(8) Å]. The ring R64(12) and R22(10) patterns of 2 and 4 (Fig. 2) represent two alternatives of hydrogen bonding network formation resembling supramolecular isomerism [61]. The involvement of uncoordinated water molecules in the formation hydrogen bonds together with coordinated water molecules resulted in the weakening of the shortest Cu···Cu distance to 8.276(2) Å for 2 and to 8.722(5) Å for 4 compared to the previous pair of complexes. On the other hand, the Cu···Cu distances of the copper atoms connected via R22(16) supramolecular synthons remain quite similar (8.762(2) Å for 2 and 8.780(5) Å for 4, respectively). The formation of these hydrogen bonded rhombohedra grids of copper(II) atoms has resulted in the layers being close together. Moreover, those grid layers are similar in that their nitrobenzoate rings protrude from the layers, thus limiting interlayer contacts. The π-π stacking contacts between two benzene rings of 2-nitrobenzoate ligands in 2 (Fig. S18) are also weaker due to the distances mentioned, and the shortest centroid-to centroid distance cg···cg is 3.75 Å [60]. The crystal structures of 2 and 4 also show weaker π-π stacking interactions between two pyridine rings of N-methylnicotinamide ligands with centroid-to centroid distances cg···cg of 3.87 Å (Fig. S19), and 3.76 Å (Fig. S20).
The molecular structure of 5 is shown in Fig. 1. The complex molecules of [Cu(4-NO2bz)2(mna)2(H2O)2] are held together with two 4-nitrobenzoic acid molecules by weak hydrogen bonds between coordinated water molecules of [Cu(4-NO2bz)2(mna)2(H2O)2] and acid molecule carboxylate oxygens O1W–H2W···O6 [O···O distance of 2.967(3) Å]. The crystal structure of 5 is given in Fig. 3.
The crystal structure of 5 consists of molecules of [Cu(4-NO2bz)2(mna)2(H2O)2] and molecules of 4-nitrobenzoic acid. The complex and the acid molecules are linked together into chains C22(13) and rings R88(42) through O–H···O and N–H···O hydrogen bonds. The chains C22(13) are created along 110 the direction by carboxamide groups of N-methylnicotinamide inserted between two 4-nitrobenzoic acid molecules, nitro groups being bonded to amide hydrogen atoms and the carboxylic groups hydrogen atoms being bonded to the carbonyl groups of solvated 4-nitrobenzoic molecules N2–H2N···O9vii and O7–H7O···O3vi [N···O distance of 3.003(3) Å and O···O distance of 2.591(3) Å; symmetry codes: (vi) − x + 1, − y + 2, − z + 2; (vii) − x, − y + 1, − z + 1]. The resulting shortest Cu···Cu distances within these chains are 14.07 Å and coplanar 4-nitrobenzoate anions of two adjacent complex molecules have centroid-to-centroid distances cg···cg of 4.88 Å. The parallel chains inserted into the adjacent chain by their carboxamide groups, form the rings R88(42) through O–H···O and N–H···O hydrogen bonds. The resulting shortest Cu···Cu distances in the 011 directions are 12.78 Å and almost rectangular grid of copper atoms is formed within this layer. The layers are stacked and held together only by weak van der Waals forces and π-π stacking contacts between the 4-nitrobenzoate ligands (the shortest centroid-to centroid distance cg···cg is 3.90 Å, Fig. S21) and between the 4-nitrobenzoic acid molecules (the shortest centroid-to centroid distance cg···cg is 3.81 Å, Fig. S22), giving the shortest interlayer Cu···Cu distances of 11.566(2) Å [60].
The principal structural features of 6 are illustrated in Fig. 1. The coordination environment of the copper atom in molecules 6 is tetragonal-bipyramidal (4+2). The tetragonal plane is built up by a pair of unidentate 3,5-dinitrobenzoate anions using carboxylate oxygen atoms [Cu–Oeq = 1.965(1) and 1.937(1) Å] in trans position, a neutral N-methylnicotinamide molecule using pyridine nitrogen atom [Cu–Neq = 2.000(1) Å] and water oxygen atom [Cu–O1W = 1.967(1) Å]. The axial positions are occupied by two other water molecules [Cu–OWax = 2.579(1) and 2.557(1) Å].
The complex 6 is a rare example of compounds with stoichiometry [Cu(RCO2)2L(H2O)3], where L is pyridine-like ligand. The molecular structure of 6 is stabilized by intramolecular hydrogen bonds (Fig. 4a) between coordinated water molecules and uncoordinated oxygen atoms of carboxylate groups of 3,5-dinitrobenzoate anions [O1W–H1W···O3 and O2W–H4W···O9 with O···O distances of 2.599(2) and 2.777(2) Å, respectively]. The complex molecules of 6 are stacked one over the other along b-axis with the coordination polyhedra slightly rotated from strictly colinear positions (Cu···Cu–O2W angle is of 19.60° and Cu···Cu–O3W angle is 23.23°) and form the chain of hydrogen-bonded molecules [O3W–H6W···O2Wxi] with O···O distances of 2.786(2) and Cu···Cu 6.894(1) Å. The rings R32(8) are created by three coordinated water molecules of two intra-chain-neighbouring complex molecules of 6 and oxygen atoms of N-methylnicotinamide of the next chain neighbouring complex molecule [O3W–H6W···O2Wxi, O2W–H3W···O1ix and O1W–H2W···O1viii with O···O distances of 2.786(2), 2.875(2) and 2.757(2) Å, respectively; symmetry codes: (viii) x, − y + 1/2, z − 1/2, (ix) x, − y + 3/2, z − 1/2; (x) − x + 1, − y, − z + 1]. This position of the neighbouring chains gives the Cu···Cu interchain distance of 8.740(3) Å and results in a rectangular grid within the layers. The slightly rotated orientation of the complex molecules is to some extent caused by a further hydrogen bond formation of the O3W atom with the neighbouring complex molecule carboxamide group. The hydrogen bonds between carboxamide groups and coordinated water molecules [N2–H2N···O3Wiii, with O···O distance of 2.976(2) Å, symmetry codes: (iii) x, y + 1, z] and hydrogen bonds O3W–H6W···O2Wx are part of rings R22(10) (Fig. 4a). The layer of molecules is linked to three-dimensional networks by multicentre hydrogen bonds [62] between coordinated water molecules O3W and one nitro groups of 3,5-dinitrobenzoate anions [O3W–H5W···O6x and O3W–H5W···O7x with O···O distances of 3.101(2) and 3.059(2) Å, respectively; symmetry codes: (x) − x + 1, − y, − z + 1] (Fig. 4b). The resulting intercopper Cu···Cu distances between neighbouring layers are 11.761(4) and 11.787(4) Å, respectively. Finally, weak π-π stacking interactions between 3,5-dinitrobenzoate ligands (the shortest centroid-to centroid distance cg···cg is 3.87 Å, Fig. S23) have been observed in the crystal structure of 6.
Hirshfeld surface analyses
Hirshfeld surface analysis was used to further study the intermolecular interactions of the crystal structures of all compounds. For the illustration, Fig. 5 shows the 3D Hirshfeld surface of 5. The 3D Hirshfeld surface of other complexes are illustrated in supplementary material (Figs. S24–S28). The 3D Hirshfeld surfaces have been mapped over dnorm shape index (Fig. 5 and Figs. S24–S28). The surfaces are shown in a transparent manner to allow visualization of the molecular moiety around which they were calculated. As shown in the Fig. 5 as well as in Figs. S24–S28, the deep red spots on the dnorm Hirshfeld surfaces indicate the close-contact interactions, which are mainly responsible for the significant intermolecular hydrogen bonding interactions. The 3D Hirshfeld surface illustration of 5 (Fig. 5) and the others (Fig. S24–S28) show the deep red areas representing O–H···O and N–H···O hydrogen bonds and also weaker C–H···O hydrogen bonding interactions.
The Hirshfeld 2D fingerprint of all compounds are illustrated in supplementary material (Figs. S29–S34). The Hirshfeld 2D fingerprint plots allow a quick and easy identification of the significant intermolecular interactions map on the molecular surface [63, 64]. As shown in Figs. S29–S34, the strong and medium H···O/O···H hydrogen bonding interactions cover the 33.5–41.6% range of the total Hirshfeld surface with two distinct spikes in the 2D fingerprint plots, indicating that the hydrogen bonding interactions are the most significant noncovalent interactions in the crystal. In addition, in the middle of scattered points in the 2D fingerplots, H···H interactions cover 16.8–41.2% range of the total Hirshfeld surface. In scattered points of the 2D fingerplots, H···C/C···H interactions cover in the range 9.3–18.3% of the total Hirshfeld surface.
EPR spectroscopy
Experimental room temperature powder EPR spectra of studied complexes exhibit features typical of an S = ½ system with axial symmetry (Fig. 6). Furthermore, the EPR spectra of 5–6 contain well resolved hyperfine splitting in a parallel part of the signal, whereas the EPR spectra of 1–4 show only the axial type of EPR line without significant hyperfine splitting. The EPR spectra were analysed using the spin Hamiltonian in an axial form [65] and the resulting simulated spin Hamiltonian parameters are summarised in Table 5.
As can be seen from Table 5, the data obtained (g⊥, g|| and A||) are typical for copper in the tetragonal bipyramidal arrangement [66]. In addition, the values of the g factors satisfy the relation g||> g⊥ > 2.0023, which is consistent with the dx2 − y2 ground electronic state. The main difference in the EPR spectra is the observation of hyperfine splitting in 5–6 in contrast with 1–4. This observation could be explained by the presence of a different structural arrangement of the paramagnetic centres in accordance with the X-ray data. The complex molecules of 1–4 are arranged in such a way that allows the copper atoms to come closer together resulting in higher half-width of the EPR signal and poorer resolution. Unlike the crystal packing in complexes 5–6 separates well the individual paramagnetic centers, allowing hyperfine splitting to be observed. Eventually, some other EPR parameters derived from the spin Hamiltonian parameters such as the average g factor gave, the geometrical parameter, G [66] and the empirical f factor [67] were also calculated and collected in the Table 5. If the value of the G parameter is G > 4 (2–5), then a negligible interaction between the Cu(II) centers is assumed [66]. On the contrary, in the case of 1 and 6 there is some exchange interaction between the copper centers as can be clearly seen from the larger half-width of the EPR signal (Fig. 6). Values of the f factor which characterises a degree of tetrahedral distortion of the tetragonal plane have been calculated for the complexes with resolved parallel hyperfine splitting (5–6) The values obtained (142 and 134 cm) support the suggestion of a square planar geometry around the copper ions with elongated tetragonal distortion [67].
Calculation of g and A constant
The EPR parameters of complexes 5–6 were further studied and analysed by means of DFT calculations. The geometry of the studied complexes (5–6) was at first optimised with the BP86 functional with Grimme dispersion correction D3BJ on the experimental geometries. Since the obtained optimised geometries show a good agreement when compared with single-crystal X-ray diffraction data, we can expect the geometry optimisation to be reasonable accurate for predicting the EPR spin Hamiltonian parameters. Using the optimised structures a molecular g and copper A tensors were computed using two different density functionals: B3LYP and TPSSh. Table 6 shows the experimental and calculated values of g⊥, g||, gave and A||. Although the calculations of the EPR parameters are done in vacuo, the calculated molecular values of g⊥ and gǀǀ well reproduce the experimentally observed relation of gǀǀ > g⊥ > 2.0023 in case of both functionals used. Analyzing the data in Table 6 we can see, that both functionals underestimate the values of g factors either gǀǀ or g⊥ whereby the trend is higher in the case of gǀǀ. A better estimate is obtained for the B3LYP functional. In any case, the DFT calculated g values are still in good qualitative agreement with the parameters obtained from the simulation of the experimental EPR spectra. This can be clearly seen by comparing the average gave values between experiment and calculation (Table 6). The underestimation of the DFT computed g values in copper (II) complexes with tetragonal symmetry has also been observed by the other authors [68, 69]. Examination of the calculated copper Aǀǀ values (Table 6) revealed very good agreement with experimental values, especially for the B3LYP functional. In this case, we obtain the value of hyperfine splitting constant |A|||= 477 and 543 MHz for 5 and 6 and experimentally extracted values are |A|||= 487 and 522 MHz. The hyperfine interaction tensor A|| is calculated as the sum of three different energy contributions: a) the contribution from the isotropic Fermi contact interaction AFC, b) the contribution from the spin-dipole interaction ASD, c) the contribution from the spin-orbital interaction ASO [44, 45]. The theoretical calculation therefore allows us to get a detailed view of how each member contributes to the resulting value of the hyperfine constant (Table 6) [70]. In the case of the 5 (B3LYP), the individual contributions were as follows: AFC = − 220 MHz, ASD = − 528 MHz and ASO = 271 MHz. Thus, the dominant contribution to the hyperfine interaction in the studied tetragonal copper(II) complexes is the contribution from the spin-dipole interaction.
Conclusion
In conclusion, we have synthesised and fully characterised six copper(II)nitrobenzoate complexes with N-methylnicotinamide. The crystal structures of the studied complexes show tetragonal bipyramidal stereochemistry around the Cu(II) ions with monodentate coordinated nitrobenzoate, N-methylnicotinamide and water ligands. The crystal packing in the crystal structures of the studied complexes shows several hydrogen-bonded supramolecular arrangements mostly due to the presence of coordinated and uncoordinated lattice water and N-methylnicotinamide molecules. In addition, Hirshfeld surface and the fingerprint plot analysis were used to quantify the contributions of each noncovalent interaction in the supramolecular structures and the final results confirm the assertion that the hydrogen bonding interactions are the most important noncovalent interactions in stabilising the net supramolecular assemblies. EPR spectra of polycrystalline samples show a monomeric signal of axial symmetry either without (1–4) or with resolved copper hyperfine splitting (5–6) for all studied complexes. The simulated spin Hamiltonian parameters are consistent with a distorted tetragonal geometry around the copper centre as was confirmed by X-ray structural data. DFT calculations of the values of the molecular g tensor and hyperfine constant A|| using B3LYP density functional show a good accordance with the experimental parameters.
References
Steed JW, Atwood JL (2000) Supramolecular chemistry. Wiley & Sons, New York
James SL (2003) Chem Soc Rev 32:276–288. https://doi.org/10.1016/j.bcp.2020.114147
Aakeröy CB, Champness NR, Janiak C (2010) CrysEngComm 12:22–43. https://doi.org/10.1039/B919819A
Furukawa S, Reboul J, Diring S, Sumida K, Kitagawa S (2014) Chem Soc Rev 43:5700–5734. https://doi.org/10.1039/c4cs00106k
Reek JNH, De Bruin B, Pullen S, Mooibroek TJ, Kluwer AM, Caumes X (2022) Chem Rev 122:12308–12369. https://doi.org/10.1021/acs.chemrev.1c00862
Seth SK, Saha I, Estarellas C, Frontera A, Kar T, Mukhopadhyay S (2011) Cryst Growth Des 11:3250–3265. https://doi.org/10.1021/cg200506q
Aakeröy CB, Beatty AM (1998) Chem Commun 10:1067–1068. https://doi.org/10.1039/a707919b
Papanikolou PA, Christidis PC, Chaviara AT, Bolos CA, Tsipis AC (2006) Eur J Inorg Chem 10:2083–2095. https://doi.org/10.1002/ejic.200500894
Beatty AM (2001) CrystEngComm 3:243–255. https://doi.org/10.1039/B109127C
Beatty AM (2003) Coord Chem Rev 246:131–143. https://doi.org/10.1016/S0010-8545(03)00120-6
Roland BK, Selby HD, Cole JR, Zheng Z (2003) Dalton Trans 22:4307–4312. https://doi.org/10.1039/B309004C
Zheng LL, Leng JD, Liu WT, Zhang WX, Lu JX, Tong ML (2008) Eur J Inorg Chem 29:4616–4624. https://doi.org/10.1002/ejic.200800486
Abu-Youssef MAM, Dey R, Gohar Y, Massoud AA, Öhrström L, Langer V (2007) Inorg Chem 46:5893–5903. https://doi.org/10.1021/ic0621594
Ma Z, Moulton B (2007) Cryst Growth Des 7:196–198. https://doi.org/10.1021/cg060602f
Ma Z, Moulton B (2007) Mol Pharm 4:373–385. https://doi.org/10.1021/mp070013k
Stachová P, Melník M, Korabik M, Mrozinski J, Koman M, Głowiak T, Valigura D (2007) Inorg Chim Acta 360:1517–1522. https://doi.org/10.1016/j.ica.2006.08.019
Moncol J, Maroszova J, Koman M, Melnik M, Valko M, Mazur M, Lis T (2008) J Coord Chem 61:3740–3752. https://doi.org/10.1080/00958970802146031
Zelenak V, Cisarova I, Llewellyn P (2007) Inorg Chem Commun 10:27–32. https://doi.org/10.1016/j.inoche.2006.08.021
Vasková Z, Moncol J, Korabik M, Valigura D, Švorec J, Lis T, Valko M, Melnik M (2010) Polyhedron 29:154–163. https://doi.org/10.1016/j.poly.2009.06.056
Brammer L, Rivas JCM, Atencio R, Fang S, Pigge FC (2000) J Chem Soc Dalton Trans 21:3855–3867. https://doi.org/10.1039/B004395H
Kozlevčar B, Leban I, Turel I, Šegedin P, Petrič M, Pohleven F, White AJP, Wiliams DJ, Sieler J (1999) Polyhedron 18:755–762. https://doi.org/10.1016/S0277-5387(98)00350-7
Kozlevčar B, Baškovič P, Arko A, Golobič A, Kitanovski N, Šegedin P (2008) Z Naturforsch Z 63b:481–488. https://doi.org/10.1515/znb-2008-0501
Sanchez-Férez F, Ejarque D, Calvet T, Font-Bardia M, Pons J (2019) Molecules 24(22):4169. https://doi.org/10.3390/molecules24224169
Qin Z, Jennings MC, Puddephatt RJ (2001) Inorg Chem 40:6220–6228. https://doi.org/10.1021/ic0106625
Moncol J, Mudra M, Lönnecke P, Hewitt M, Valko M, Morris H, Švorec J, Melnik M, Mazur M, Koman M (2007) Inorg Chim Acta 360:3213–3225. https://doi.org/10.1016/j.ica.2007.03.027
Kuchtanin V, Moncol J, Mrozinski J, Kalinska B, Padelková Z, Švorec J, Segľa P, Melnik M (2013) Polyhedron 50:546–555. https://doi.org/10.1016/j.poly.2012.11.041
Jozefíková F, Kucková L, Lokaj J, Mazúr M, Moncol J (2020) Chem Pap 74:3727–3740. https://doi.org/10.1007/s11696-020-01224-z
Halaška J, Lokaj J, Jomová K, Ružičková Z, Mazúr M, Moncol J (2020) Polyhedron 175:114237. https://doi.org/10.1016/j.poly.2019.114237
Puchoňová M, Repická Z, Moncol J, Ružičková Z, Mazúr M, Valigura D (2015) J Mol Struct 1092:1–8. https://doi.org/10.1016/j.molstruc.2015.02.086
Vasková Z, Kitanovski N, Jogličič Z, Strach P, Ružičková Z, Mazúr M, Koman M, Kozlevčar B, Moncol J (2014) Polyhedron 81:555–563. https://doi.org/10.1016/j.poly.2014.07.017
Ozarowski Andrzej, Spin Software (SpinP.exe). Available online: https://nationalmaglab.org/user-facilities/emr/software/. Accessed 5 Jan 2016
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) J Appl Crystallogr 48:3–10. https://doi.org/10.1107/S1600576714022985
Sheldrick GM (2008) Acta Crystallogr Sect A 64:112–122. https://doi.org/10.1107/S0108767307043930
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, Giacovazzo C, Mallamo M, Mazzone A, Polidori G, Spagna R (2012) J Appl Crystallogr 45:357–361. https://doi.org/10.1107/S0021889812001124
Sheldrick GM (2015) Acta Crystallogr Sect C 71:3–8. https://doi.org/10.1107/S2053229614024218
Bourhis LJ, Dolomanov OV, Gildea RJ, Howard JAK, Puschman H (2015) Acta Crystallogr Sect A 71:59. https://doi.org/10.1107/S2053273314022207
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschman H (2009) J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Macraw CF, Sovago I, Cattrell SJ, Galek PTA, McCabe P, Pidcock M, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) J Appl Crystallogr 53:226–235. https://doi.org/10.1107/S1600576719014092
Spackman PR, Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Jayalitaka D, Spackman MA (2021) J Appl Crystallogr 54:1006–1011. https://doi.org/10.1107/S1600576721002910
Hirshfeld FL (1977) Theor Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Spackman MA, Jayalitaka D (2009) CrystEngComm 11:19–32. https://doi.org/10.1039/B818330A
Spackman MA, McKinnon JJ (2002) CrystEngComm 4:378–392. https://doi.org/10.1039/B203191B
Parkin A, Barr G, Dong W, Gilmore CJ, Jayalitaka D, McKinnon JJ, Spackman MA, Wilson CC (2007) CrystEngComm 9:648–652. https://doi.org/10.1039/B704177B
Neese F (2012) Wiley Interdiscip Rev Comput Mol Sci 2:73–78. https://doi.org/10.1002/wcms.81
Neese F (2003) J Chem Phys 118:3939. https://doi.org/10.1063/1.1540619
Neese F (2001) J Chem Phys 115:11080. https://doi.org/10.1063/1.1419058
Hedegard ED, Kongsted J, Sauer SPA (2011) J Chem Theory Comput 7:4077–4087. https://doi.org/10.1021/ct200587k
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/b508541a
Weigend F (2006) Phys Chem Chem Phys 8:1057–1065. https://doi.org/10.1039/B515623H
Neese F, Wennmohs F, Hansen A, Becker U (2009) Chem Phys 356:98–109. https://doi.org/10.1016/j.chemphys.2008.10.036
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds, Part B, 6th edn. John Wiley and Sons, Inc., Hoboken, pp 1–199
Kumar S, Sharma RP, Venugopalan P, Gondil VS, Chhibber S, Aree T, Witwicki M, Ferretti V (2018) Inorg Chim Acta 469:288–297. https://doi.org/10.1016/j.ica.2017.09.032
Sharma RP, Saini A, Monga D, Venugopalan P, Jezierska J, Ozarowski A, Ferretti V (2014) New J Chem 38:437–447. https://doi.org/10.1039/c3nj00736g
Hathaway BJ, Billing DE (1970) Coord Chem Rev 5:143. https://doi.org/10.1016/S0010-8545(00)80135-6
Piroš M, Schoeller M, Koňariková K, Valentová J, Švorc L’, Moncol’ J, Valko M, Švorec J (2023) Inorganics 11:108. https://doi.org/10.3390/inorganics11030108
Seddon KR (2004) Cryst Growth Des 4:1087. https://doi.org/10.1021/cg030084y
Nangia A (2006) Cryst Growth Des 6(1):32–35. https://doi.org/10.1021/cg050396w
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555–1573. https://doi.org/10.1002/anie.199515551
Bujdošová Z, Kuchár J, Györyová K (2010) Acta Crystallogr Sect E Struct Rep Online 66:394–395. https://doi.org/10.1107/S1600536810008834
Janiak C (2000) J Chem Soc Dalton Trans 21:3885–3896. https://doi.org/10.1039/B003010O
Zhang JP, Huang XC, Chen XM (2009) Chem Soc Rev 38:2385–2396. https://doi.org/10.1039/B900317G
Gilli G, Gilli P (2009) The nature of the hydrogen bond: outline of a comprehensive hydrogen bond theory. Oxford University Press Inc, New York
McKinnon JJ, Spackman MA, Mitchell AS (2004) Acta Crystallogr Sect B 60:627–668. https://doi.org/10.1107/S0108768104020300
McKinnon JJ, Jayalitaka D, Spackman MA (2007). Chem Commun. https://doi.org/10.1039/B704980C
Weil A, Bolton JR, Wertz JE (2007) Electron paramagnetic resonance spectroscopy: elementary theory and practical applications. Wiley-Interscience, New York
Hathaway BJ, Tomlinson AAG (1970) Coord Chem Rev 5:1–43. https://doi.org/10.1016/S0010-8545(00)80073-9
Sakaguchi U, Addison AW (1979). J Chem Soc Dalton Trans. https://doi.org/10.1039/DT9790000600
Ames WM, Larsen SC (2009) J Phys Chem A 113:4305–4312. https://doi.org/10.1021/jp810924j
Guillou A, Lima LMP, Roger M, Esteban-Gomez D, Delgado R, Platas-Iglesias C, Patinec V, Tripier R (2017) Eur J Inorg Chem 2017:2435–2443. https://doi.org/10.1002/ejic.201700176
Sciortino G, Lubino G, Marechal JD, Garribba E (2018) Magnetochemistry 4:55. https://doi.org/10.3390/magnetochemistry4040055
Acknowledgements
Slovak grant agencies (VEGA 1/0482/20, VEGA 1/0686/23, APVV-19-0087, APVV-18-0016) are acknowledged for the financial support. Thanks to the Ministry of Education, Science, Research and Sport of the Slovak Republic for funding within the scheme “Excellent research teams”. We are also grateful to the HPC center at the Slovak University of Technology in Bratislava, which is a part of the Slovak Infrastructure of High Performance Computing (SIVVP project, ITMS code 26230120002, funded by the European region development funds, ERDF), for the computational time and resources made available. This publication was also created on the basis of the major project “Advancing University Capacity and Competence in Research, Development and Innovation“ (ITMS project code: 313021X329) supported by Operational Programme Integrated Infrastructure and funded by the European Regional Development Fund.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
M.P. and Z.V did synthesis and spectral characterization. J.M. did structural characterization. M.M. did EPR measurements. J.Š did DFT calculations and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piroš, M., Vasková, Z., Mazúr, M. et al. Hydrogen-bonded supramolecular structures in copper(II) nitrobenzoates with N-methylnicotinamide: synthesis, supramolecular structure, Hirshfeld surface analysis, spectral and DFT study. Transit Met Chem 48, 281–295 (2023). https://doi.org/10.1007/s11243-023-00542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-023-00542-x