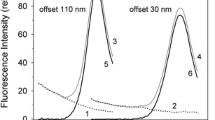
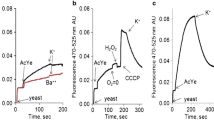
Abstract
The fluorescent dye 3,3′-dipropylthiadicarbocyanine, diS-C3(3), is a suitable probe to monitor real changes of plasma membrane potential in yeast cells which are too small for direct membrane potential measurements with microelectrodes. A method presented in this paper makes it possible to convert changes of equilibrium diS-C3(3) fluorescence spectra, measured in yeast cell suspensions under certain defined conditions, into underlying membrane potential differences, scaled in the units of millivolts. Spectral analysis of synchronously scanned diS-C3(3) fluorescence allows to assess the amount of dye accumulated in cells without otherwise necessary sample taking and following separation of cells from the medium. Moreover, membrane potential changes can be quantified without demanding calibration protocols. The applicability of this approach was demonstrated on the depolarization of Rhodotorula glutinis yeast cells upon acidification of cell suspensions and/or by increasing extracellular K+ concentration.
Similar content being viewed by others
References
Bakker R, Dobbelmann J, Borst-Pauwels GWFH (1986) Membrane potential in the yeast Endomyces magnusii measured by microelectrodes and TPP+ distribution. Biochim Biophys Acta 861:205–209
Bashford CL (1981) The measurement of membrane potential using optical indicators. Biosci Rep 1:183–196
Borst-Pauwels GWFH (1989) Ion transport in yeast including lipophilic ions. Methods Enzymol 174:603–616
Cohen LB, Salzberg BM (1978) Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol 83:35–88
Cossarizza A, Ceccarelli D, Masini A (1996) Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res 222:84–94
Criddle RS, Schatz G (1969) Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry 8:322–334
Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM (1988) Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J 53:785–794
Freedman JC, Hoffman JF (1979) The relation between dicarbocyanine dye fluorescence and the membrane potential of human red blood cells set at varying Donnan equilibria. J Gen Physiol 74:187–212
Friedman MH (2008) Principles and models of biological transport. Springer, New York, p 508
Gage RA, Vanwijngaarden W, Theuvenet APR, Borst-Pauwels GWFH, Verkleij AJ (1985) Inhibition of Rb+ uptake in yeast by Ca2+ is caused by a reduction in the surface potential and not in the Donnan potential. Biochim Biophys Acta 812:1–8
Gaskova D, Brodska B, Herman P et al (1998) Fluorescent probing of membrane potential in walled cells: diS-C-3(3) assay in Saccharomyces cerevisiae. Yeast 14:1189–1197
Gross D, Loew LM (1989) Fluorescent indicators of membrane potential: microspectrofluorometry and imaging. Methods Cell Biol 30:193–218
Hauer R, Hofer M (1978) Evidence for interactions between the energy-dependent transport of sugars and the membrane potential in the yeast Rhodotorula gracilis (Rhodosporidium toruloides). J Membr Biol 43:335–349
Hauer R, Uhlemann G, Neumann J, Hofer M (1981) Proton pumps of the plasmalemma of the yeast Rhodotorula gracilis—their coupling to fluxes of potassium and other ions. Biochim Biophys Acta 649:680–690
Hladky SB, Rink TJ (1976) Potential difference and the distribution of ions across the human red blood cell membrane; a study of the mechanism by which the fluorescent cation, diS-C3-(5) reports membrane potential. J Physiol 263:287–319
Hockings PD, Rogers PJ (1996) The measurement of transmembrane electrical potential with lipophilic cations. Biochim Biophys Acta 1282:101–106
Hofer M, Kunemund A (1985) Tetraphenylphosphonium ion is a true indicator of negative plasma-membrane potential in the yeast Rhodotorula glutinis. Experiments under osmotic stress and at low external pH values. Biochem J 225:815–819
Hofer M, Novacky A (1986) Measurement of plasma membrane potentials of yeast cells with glass microelectrodes. Biochim Biophys Acta 862:372–378
Hofer M, Nicolay K, Robillard G (1985) The electrochemical H+ gradient in the yeast Rhodotorula glutinis. J Bioenerg Biomem 17:175–182
Hoffman JF, Laris PC (1974) Determination of membrane potentials in human and Amphiuma red blood cells by means of a fluorescent probe. J Physiol 239:519–552
Kaji DM (1993) Effect of membrane potential on K-Cl transport in human erythrocytes. Am J Physiol 239(264):C376–C382
Krasznai Z, Marian T, Balkay L, Emri M, Tron L (1995) Flow cytometric determination of absolute membrane potential of cells. J Photochem Photobiol B 28:93–99
Labajova A, Vojtiskova A, Krivakova P, Kofranek J, Drahota Z, Houstek J (2006) Evaluation of mitochondrial membrane potential using a computerized device with a tetraphenylphosphonium-selective electrode. Anal Biochem 353:37–42
Ling G, Gerard RW (1949) The normal membrane potential of frog Sartorius fibers. J Cell Compart Physiol 34:383–396
Lloyd JBF (1971) Synchronized excitation of fluorescence emission spectra. Nat Phys Sci 231:64–65
Loew LM, Tuft RA, Carrington W, Fay FS (1993) Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J 65:2396–2407
Lolkema JS, Hellingwerf KJ, Konings WN (1982) The effect of ‘probe binding’ on the quantitative determination of the proton-motive force in bacteria. Biochim Biophys Acta 681:85–94
Macey RI, Adorante JS, Orme FW (1978) Erythrocyte membrane potentials determined by hydrogen ion distribution. Biochim Biophys Acta 512:284–295
Mao C, Kisaalita WS (2004) Determination of resting membrane potential of individual neuroblastoma cells (IMR-32) using a potentiometric dye (TMRM) and confocal microscopy. J Fluoresc 14:739–743
Maresova L, Urbankova E, Gaskova D, Sychrova H (2006) Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res 6:1039–1046
Maresova L, Muend S, Zhang YQ, Sychrova H, Rao R (2009) Membrane hyperpolarization drives cation influx and fungicidal activity of amiodarone. J Biol Chem 284:2795–2802
Pena A, Sanchez NS, Calahorra M (2010) Estimation of the electric plasma membrane potential difference in yeast with fluorescent dyes: comparative study of methods. J Bioenerg Biomem 42:419–432
Pereira MBP, Tisi R, Fietto LG et al (2008) Carbonyl cyanide m-chlorophenylhydrazone induced calcium signaling and activation of plasma membrane H+ -ATPase in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 8:622–630
Plasek J, Hrouda V (1991) Assessment of membrane potential changes using the carbocyanine dye, diS-C3(5): synchronous excitation spectroscopy studies. Eur Biophys J 19:183–188
Plasek J, Sigler K (1996) Slow fluorescent indicators of membrane potential: a survey of different approaches to probe response analysis. J Photochem Photobiol B 33:101–124
Plasek J, Dale RE, Sigler K, Laskay G (1994) Transmembrane potentials in cells: a diS-C3(3) assay for relative potentials as an indicator of real changes. Biochim Biophys Acta 1196:181–190
Plasek J, Gaskova D, Vecer J, Sigler K (2000) Use of synchronously excited fluorescence to assess the accumulation of membrane potential probes in yeast cells. Folia Microbiol 45:225–229
Przybylo M, Borowik T, Langner M (2010) Fluorescence techniques for determination of the membrane potentials in high throughput screening. J Fluoresc 20:1139–1157
Ross MF, Kelso GF, Blaikie FH et al (2005) Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 70:222–230
Shapiro HM (1994) Cell membrane potential analysis. Methods Cell Biol 41:121–133
Sims PJ, Waggoner AS, Wang CH, Hoffman JF (1974) Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 13:3315–3330
Smith JC (1990) Potential-sensitive molecular probes in membranes of bioenergetic relevance. Biochim Biophys Acta 1016:1–28
Stevens HC, Nichols JW (2007) The proton electrochemical gradient across the plasma membrane of yeast is necessary for phospholipid flip. J Biol Chem 282:17563–17567
Sundelacruz S, Levin M, Kaplan DL (2009) Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev 5:231–246
Theuvenet APR, Borst-Pauwels GWFH (1983) Effect of surface potential on Rb + uptake in yeast. The effect of pH. Biochim Biophys Acta 734:62–69
Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A (1984) Ammonium uptake in Lemna gibba G 1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant 61:369–376
Waggoner A (1976) Optical probes of membrane potential. J Membr Biol 27:317–334
Waggoner AS (1979) Dye indicators of membrane potential. Annu Rev Biophys Bioeng 8:47–68
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Theory of diS-C3(3) fluorescence response to membrane potential changes in yeast
Implications of the Nernst equation (Plasek et al. 1994)
The standard interpretation of the relationship between membrane potential and the uptake of small lipophilic ions by cells and/or cell organelles is based on the assumption that the equilibrium concentrations of unbound ions in cells, cin, and cell suspension medium, cout, obey the Nernst equation (Ehrenberg et al. 1988; Krasznai et al. 1995; Loew et al. 1993; Lolkema et al. 1982; Plasek and Hrouda 1991; Ross et al. 2005). For diS-C3(3) assays relying on the spectral unmixing of free- and bound-dye contributions from complex fluorescence spectra of cell suspensions it is essential to use dye concentrations low enough to guarantee that the amount of intracellular bound dye is directly proportional to the intracellular free dye concentration resulting from the Nernstian accumulation of diS-C3(3) in the cells (Plasek et al. 1994), see Methods.
Moreover, we will assume that the complex fluorescence of diS-C3(3) stained cell suspensions is dominated by the fluorescence of free dye dissolved in the cell suspension medium, Ff, and the fluorescence of dye molecules bound to cytosolic proteins, or other intracellular substances, Fb, while both the contribution of fluorescence of unbound dye molecules present in the cell cytosol, Fin, and fluorescence of dye molecules bound to the outer cell surface, F outb , is negligible in properly designed experiments. In particular, Fin is negligible compared to Ff if the partial volume of cells in measured suspensions is sufficiently low, what is the case in present experimental protocols. The amount of dye bound to the outer cell surface can also be neglected compared to the amount of the dye bound to cytosolic proteins. This was proved by fluorescence microscopy, which revealed no bright rim around the fluorescent images of stained cells.
Then, following the model presented in (Plasek et al. 1994), the ratio of bound and free-dye fluorescence intensities measured with spectral unmixing, i.e., Fb and Ff, respectively, can be combined with the Nernst equation to yield
where the physical constants R, T and F have their usual meaning, ΔΨ denotes the membrane potential. A number of multiplicative factors involved in this equation represents following experimental parameters: partial volume of cells (Vc) and cell suspension medium (Vs) within an effective fluorescence detection volume inside a cuvette, fluorescence quantum yields (Qb and Qf) and instrument sensitivity factors (Sb and Sf) of bound and free-dye fluorescence, respectively, and kb is a proportionality constant of the ratio between the bound and free-dye concentrations inside the cells. For simplicity, the pre-exponential fraction in Eq. A1 can be represented by a single constant K bf . To highlight quantitative aspects of this equation, we have introduced a voltage factor URTF = RT/F, the value of which is 25.7−26.9 mV at physiological temperatures between 25 and 37 °C.
Despite the impossibility to determine the actual value of the Kbf coefficient in real experiments Eq. A1 can be still used for the assessment of membrane potential differences in certain defined situations. In particular, this is the case, when it is justified to postulate that the cell-related parameters Vc and kb remain constant on going from a cell physiological state 1 (characterized by membrane potential ΔΨ1) to another state 2 (characterized by membrane potential ΔΨ2). Upon a simple logarithmic transformation of Eq. A1 we get finally
These two equations can be combined into the following simple formula, in which the unknown constant Kbf has been cancelled out:
In this way, the Fb/Ff fluorescence ratios obtained by the spectral unmixing of diS-C3(3) fluorescence spectra measured in cell suspensions can be used to quantify membrane potential differences in the absolute scale of mV, without necessity to perform any a priori calibration experiment.
However, the Eq. A3 is obviously an oversimplification aimed merely to illustrate the idea of a quantitative link between the diS-C3(3) fluorescence spectra and membrane potential changes of examined cells. To match real experimental conditions, the above model must be amended by including i) the uptake of diS-C3(3) by mitochondria, ii) the effect of yeast cell wall and surface potential, and iii) the extrusion of diS-C3(3) from yeast cells by MDR pumps, responsible for multi drug resistance of yeast cells.
Contribution of probe accumulation in mitochondria
The treatment of the contribution of mitochondria to the diS-C3(3) accumulation in yeast cell follows also the model already used for animal cells (Plasek et al. 1994), which leads to a conclusion that the correction of Eq. A1 for the contribution of mitochondria can be represented by a multiplicative factor M(Vm, ΔΨm), which is the function of both the partial volume of mitochondria in cells, Vm, and mitochondrial membrane potential, ΔΨm.
This means that Eq. A3 can be still used for assessment of membrane potential differences whenever the postulation is justified that neither the partial volume of mitochondria in the cells, nor the mitochondrial membrane potential vary in the course of the experiment. The role of mitochondria-related artefacts in membrane potential assays can also be significantly lessened by working with fermenting yeast cells in which the function of the mitochondria is highly reduced (Criddle and Schatz 1969). On the other hand, experimental protocols based on uncoupling yeast mitochondria with protonophores are not recommended since these protonophores, such as carbonyl cyanide m-chlorophenylhydrazone (CCCP), collapse inevitably the proton electrochemical gradient across the plasma membrane as well (Macey et al. 1978; Pereira et al. 2008; Stevens and Nichols 2007).
The role of cell wall and surface potential
The effect of cell wall, which is specific for yeast and bacteria, has not been treated in models published in our preceding papers. Since the cell wall space of yeast possess negative Donnan potential, a correction should be made for the accumulation of cationic species in the close proximity of cell membrane (Borst-Pauwels 1989; Theuvenet and Borst_Pauwels 1983), albeit under some conditions the role of cation accumulation in the cell wall might be rather small (Gage et al. 1985).
As the Nernstian accumulation of diS-C3(3) in yeast cells is concerned, a true dye concentration at the periplasm/plasmalemma interface, cppi, must be therefore inserted into the Nernst equation instead of its bulk concentration, cout, in the cell suspension medium. The former concentration is proportional to the bulk diS-C3(3) concentration through two distinct exponential factors that arise from i) the partition coefficient of diS-C3(3) between the aqueous suspension medium and the less polar cell wall space, and ii) the series of Boltzmann equilibriums related to the Donnan potential of cell wall and the surface potential of plasmalemma. It seems therefore practical to introduce a certain “effective“negative surface potential ψ*, which makes it possible to express the increase of local diS-C3(3) concentration at membrane surface cppi with respect to its bulk value cout in terms of a product cout exp(ψ*/URTF). Then the Nernst equation used to describe the accumulation of diS-C3(3) in animal cells (Plasek et al. 1994), i.e.,
must be replaced by
and Eq. A3 modified accordingly to yield a following equation
which reflects a fact that the intracellular accumulation of small lipophilic cations does not respond correctly to membrane potential changes if the surface potential of examined cells varies in the course of the experiment. This is a very important, thought often ignored, aspect of monitoring cell membrane potentials by using lipophilic cations. In particular, the use of different buffers may result in serious discrepancies between diS-C3(3) fluorescence intensities measured in apparently parallel assays, just because the surface potential of cell membranes is extremely sensitive to the ionic strength of cell suspension medium. On the other hand, when the ionic strength is maintained constant during the experiment, [ψ2*− ψ1*] becomes zero and thus can be omitted
Contribution of MDR pumps
Voltage-sensing fluorescent dyes are possible substrates of MDR pumps responsible for multi drug resistance of yeast cells. When considering the effect of these pumps on dye redistribution it is necessary to move from the thermodynamic equilibrium model to a steady-state equilibrium, under which the dye uptake by cells is counter balanced by the MDR pump mediated outward flux JMDR (in mol m−1 s−1). The transmembrane inward flux of ions J (in mol m−1 s−1) that is driven by the membrane potential can be expressed
where G is the membrane conductivity, z the ion valence, and other symbols as above (Friedman 2008). In the steady state this flux is offset by JMDR, so that J = JMDR. Under these circumstances Eq. A8 should be replaced by the following formula
This implicates that the cationic dye redistribution reflects false membrane potentials that are reduced with respect to their true values by a term \( \Delta {\Psi^{{MDR}}} = \frac{{zF}}{G}{J_{{MDR}}} \) and thus, Eq. A3 must be extended to a more complex formula
Unfortunately, little is known about the size of MDR mediated currents. This hampers both the qualitative and quantitative evaluation of the membrane potential dependent response of slow dyes. Therefore, Eq. A10 can be used for the assessment of membrane potential changes only in a few special cases, such as when working with MDR pump-deficient yeast mutants and/or with cells for which it was demonstrated that the actual contribution of MDR pumps to membrane potential, ΔΨ MDR, stays constant and independent of the rate of diS-C3(3) accumulation in cells (see Methods). Under the latter conditions, the term \( \left( {\Delta \Psi_2^{{MDR}} - \Delta \Psi_1^{{MDR}}} \right) \) of Eq. A10 is cancelled out and thus, true ΔΨ2 – ΔΨ1 differences can be obtained.
Important protocol requirements for quantifying membrane potential changes
The most important requirements for the quantitative evaluation of diS-C3(3) fluorescence response to membrane potential changes in mV are:
-
a)
It is essential to use dye concentrations low enough to guarantee direct proportionality between the amount of intracellular bound dye and the intracellular free dye concentration resulting from the Nernstian accumulation of diS-C3(3) in the cells.
-
b)
Any experimental protocol aimed at the assessment of membrane potential changes between two physiological states of examined cell suspension must guarantee that the steady-state dye redistribution between cells and their medium was reached. This does not apply when the time-course of membrane potential changes is monitored.
-
c)
All details of the experiment must be carefully controlled so that no variations occur in the density of cells in cell suspension or in ionic strength of the suspension medium.
-
d)
Ideal situation for the quantitative evaluation of diS-C3(3) fluorescence in terms of underlying membrane potential changes is represented by experiments in which the cells respond to any stimulus in periods short enough from the point of view of possible changes in cellular parameters such as metabolism, protein synthesis, macromolecular composition of cytosol as well as partial volume of mitochondria and mitochondrial membrane potential.
Rights and permissions
About this article
Cite this article
Plášek, J., Gášková, D., Lichtenberg-Fraté, H. et al. Monitoring of real changes of plasma membrane potential by diS-C3(3) fluorescence in yeast cell suspensions. J Bioenerg Biomembr 44, 559–569 (2012). https://doi.org/10.1007/s10863-012-9458-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9458-8