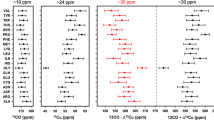
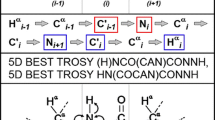
Abstract
Sequential resonance assignment strategies are typically based on matching one or two chemical shifts of adjacent residues. However, resonance overlap often leads to ambiguity in resonance assignments in particular for intrinsically disordered proteins. We investigated the potential of establishing connectivity through the three-bond couplings between sequentially adjoining backbone carbonyl carbon nuclei, combined with semi-constant time chemical shift evolution, for resonance assignments of small folded and larger unfolded proteins. Extended sequential connectivity strongly lifts chemical shift degeneracy of the backbone nuclei in disordered proteins. We show here that 3D (H)N(COCO)NH and (HN)CO(CO)NH experiments with relaxation-optimized multiple pulse mixing correlate up to seven adjacent backbone amide nitrogen or carbonyl carbon nuclei, respectively, and connections across proline residues are also obtained straightforwardly. Multiple, recurrent long-range correlations with ultra-high resolution allow backbone 1HN, 15NH, and 13C′ resonance assignments to be completed from a single pair of 3D experiments.













Similar content being viewed by others
Abbreviations
- 3 J C′C′ :
-
Three-bond coupling between sequentially adjoining 13C′ nuclei
- αSyn:
-
α-Synuclein
- CSA:
-
Chemical shift anisotropy
- DSS:
-
4,4-Dimethyl-4-silapentane-1-sulfonate
- IDP:
-
Intrinsically disordered protein
- MOCCA-XY16:
-
Modified phase cycled Carr–Purcell multiple pulse sequence with XY16 supercycles
- RF:
-
Radiofrequency
References
Balayssac S, Jimenez B, Piccioli M (2006) 13C direct detected COCO-TOCSY: a tool for sequence specific assignment and structure determination in protonless NMR experiments. J Magn Reson 182:325–329
Bermel W, Bertini I, Duma L, Felli IC, Emsley L, Pierattelli R, Vasos PR (2005) Complete assignment of heteronuclear protein resonances by protonless NMR spectroscopy. Angew Chem Int Ed 44:3089–3092
Bermel W, Bertini I, Felli IC, Lee YM, Luchinat C, Pierattelli R (2006) Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc 128:3918–3919
Bermel W, Bertini I, Felli IC, Gonnelli L, Kozminski W, Piai A, Pierattelli R, Stanek J (2012) Speeding up sequence specific assignment of IDPs. J Biomol NMR 53:293–301
Buevich AV, Baum J (1999) Dynamics of unfolded proteins: incorporation of distributions of correlation times in the model free analysis of NMR relaxation data. J Am Chem Soc 121:8671–8672
Buevich AV, Shinde UP, Inouye M, Baum J (2001) Backbone dynamics of the natively unfolded pro-peptide of subtilisin by heteronuclear NMR relaxation studies. J Biomol NMR 20:233–249
Camilloni C, De Simone A, Vranken WF, Vendruscolo M (2012) Determination of secondary structure populations in disordered states of proteins using nuclear magnetic resonance chemical shifts. Biochemistry 51:2224–2231
Chang SL, Tjandra N (2005) Temperature dependence of protein backbone motion from carbonyl 13C and amide 15N NMR relaxation. J Magn Reson 174:43–53
Clubb RT, Thanabal V, Wagner G (1992) A constant-time three-dimensional triple-resonance pulse scheme to correlate intraresidue 1HN, 15N, and 13C′ chemical shifts in 15N–13C-labeled proteins. J Magn Reson 97:213–217
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Engelke J, Ruterjans H (1997) Backbone dynamics of proteins derived from carbonyl carbon relaxation times at 500, 600 and 800 MHz: application to ribonuclease T1. J Biomol NMR 9:63–78
Felli IC, Pierattelli R (2014) Novel methods based on 13C detection to study intrinsically disordered proteins. J Magn Reson 241:115–125
Felli IC, Pierattelli R, Glaser SJ, Luy B (2009) Relaxation-optimised Hartmann–Hahn transfer using a specifically Tailored MOCCA-XY16 mixing sequence for carbonyl–carbonyl correlation spectroscopy in 13C direct detection NMR experiments. J Biomol NMR 43:187–196
Furrer J, Kramer F, Marino JP, Glaser SJ, Luy B (2004) Homonuclear Hartmann–Hahn transfer with reduced relaxation losses by use of the MOCCA-XY16 multiple pulse sequence. J Magn Reson 166:39–46
Giehm L, Svergun DI, Otzen DE, Vestergaard B (2011) Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proc Natl Acad Sci U S A 108:3246–3251
Grzesiek S, Bax A (1992) Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J Am Chem Soc 114:6291–6293
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N-enriched proteins. J Biomol NMR 3:185–204
Grzesiek S, Bax A (1997) A three-dimensional NMR experiment with improved sensitivity for carbonyl–carbonyl J correlation in proteins. J Biomol NMR 9:207–211
Hu JS, Bax A (1996) Measurement of three bond 13C–13C J couplings between carbonyl and carbonyl/carboxyl carbons in isotopically enriched proteins. J Am Chem Soc 118:8170–8171
Ikura M, Kay LE, Bax A (1990) A novel approach for sequential assignment of proton, carbon-13, and nitrogen-15 spectra of larger proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry 29:4659–4667
Kadkhodaie M, Rivas O, Tan M, Mohebbi A, Shaka AJ (1991) Broadband homonuclear polarization using flip–flop spectroscopy. J Magn Reson 91:437–443
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kay LE, Xu GY, Yamazaki T (1994) Enhanced-sensitivity triple-resonance spectroscopy with minimal H2O saturation. J Magn Reson A 109:129–133
Kjaergaard M, Poulsen FM (2012) Disordered proteins studied by chemical shifts. Prog Nucl Magn Reson Spectrosc 60:42–51
Kosol S, Contreras-Martos S, Cedeno C, Tompa P (2013) Structural characterization of intrinsically disordered proteins by NMR spectroscopy. Molecules 18:10802–10828
Kramer F, Peti W, Griesinger C, Glaser SJ (2001) Optimized homonuclear Carr–Purcell-type dipolar mixing sequence. J Magn Reson 149:58–66
Lipari G, Szabo A (1982a) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559
Lipari G, Szabo A (1982b) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 2. Analysis of experimental results. J Am Chem Soc 104:4559–4570
Liu A, Riek R, Wider G, von Schroetter C, Zahn R, Wüthrich K (2000) NMR experiments for resonance assignments of 13C, 15N doubly-labeled flexible polypeptides: application to the human prion protein hPrP(23–230). J Biomol NMR 16:127–138
Logan TM, Olejniczak ET, Xu RX, Fesik SW (1993) A general method for assigning NMR spectra of denatured proteins using 3D HC(CO)NH-TOCSY triple resonance experiments. J Biomol NMR 3:225–231
Maltsev AS, Ying J, Bax A (2012) Deuterium isotope shifts for backbone 1H, 15N and 13C nuclei in intrinsically disordered protein and α-synuclein. J Biomol NMR 54:181–191
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399
Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wuthrich K (1998) Recommendations for the presentation of NMR structures of proteins and nucleic acids. IUPAC–IUBMB–IUPAB inter-union task group on the standardization of data bases of protein and nucleic acid structures determined by NMR spectroscopy. J Biomol NMR 12:1–23
Markwick PR, Sattler M (2004) Site-specific variations of carbonyl chemical shift anisotropies in proteins. J Am Chem Soc 126:11424–11425
Motackova V, Novacek J, Zawadzka-Kazimierczuk A, Kazimierczuk K, Zidek L, Sanderova H, Krasny L, Kozminski W, Sklenar V (2010) Strategy for complete NMR assignment of disordered proteins with highly repetitive sequences based on resolution-enhanced 5D experiments. J Biomol NMR 48:169–177
Mulder FAA, Lundqvist M, Scheek RM (2010) Nuclear magnetic resonance spectroscopy applied to (intrinsically) disordered proteinsin. In: Uversky VN, Longhi S (eds) Instrumental analysis of intrinsically disordered proteins: assessing structure and conformation. Wiley, Hoboken, pp 61–87
Mulder FAA, Otten R, Scheek RM (2011) Origin and removal of mixed-phase artifacts in gradient sensitivity enhanced heteronuclear single quantum correlation spectra. J Biomol NMR 51:199–207
Novacek J, Zidek L, Sklenar V (2014) Toward optimal-resolution NMR of intrinsically disordered proteins. J Magn Reson 241:41–52
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34:93–158
Schwarzinger S, Kroon GJ, Foss TR, Chung J, Wright PE, Dyson HJ (2001) Sequence-dependent correction of random coil NMR chemical shifts. J Am Chem Soc 123:2970–2978
Shaka AJ, Lee CJ, Pines A (1988) Iterative schemes for bilinear operatiors; application to spin decoupling. J Magn Reson 77:274–293
Tamiola K, Mulder FAA (2012) Using NMR chemical shifts to calculate the propensity for structural order and disorder in proteins. Biochem Soc Trans 40:1014–1020
Tamiola K, Acar B, Mulder FAA (2010) Sequence-specific random coil chemical shifts of intrinsically disordered proteins. J Am Chem Soc 132:18000–18003
Uversky VN (2011) Intrinsically disordered proteins from A to Z. Int J Biochem Cell Biol 43:1090–1103
Uversky VN, Fink AL (2004) Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta 1698:131–153
Wang T, Cai S, Zuiderweg ERP (2003) Temperature dependence of anisotropic protein backbone dynamics. J Am Chem Soc 125:8639–8643
Wishart DS, Bigam CG, Holm A, Hodges RS, Sykes BD (1995) 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J Biomol NMR 5:67–81
Wright PE, Dyson HJ (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 293:321–331
Yao J, Dyson HJ, Wright PE (1997) Chemical shift dispersion and secondary structure prediction in unfolded and partly folded proteins. FEBS Lett 419:285–289
Ying J, Roche J, Bax A (2014) Homonuclear decoupling for enhancing resolution and sensitivity in NOE and RDC measurements of peptides and proteins. J Magn Reson 241:97–102
Yuwen T, Skrynnikov NR (2014a) CP-HISQC: a better version of HSQC experiment for intrinsically disordered proteins under physiological conditions. J Biomol NMR 58:175–192
Yuwen T, Skrynnikov NR (2014b) Proton-decoupled CPMG: a better experiment for measuring 15N R2 relaxation in disordered proteins. J Magn Reson 241:155–169
Acknowledgments
We thank Camilla B. Andersen and Tania A. Nielsen (Aarhus University, Denmark) for the expression and purification of αSyn and ubiquitin, respectively. Y.Y. is supported by an EMBO Long-Term Fellowship (ALTF 687-2013).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshimura, Y., Kulminskaya, N.V. & Mulder, F.A.A. Easy and unambiguous sequential assignments of intrinsically disordered proteins by correlating the backbone 15N or 13C′ chemical shifts of multiple contiguous residues in highly resolved 3D spectra. J Biomol NMR 61, 109–121 (2015). https://doi.org/10.1007/s10858-014-9890-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-014-9890-7