Abstract
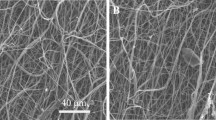
The major challenge to treat the clinical adverse effects of long-segment urethra is in achieving viable tissue substitution. The substituted construct’s properties-such as its resilience, contraction, and ability to minimize scar-stenosis formation should be considered. In the present work, a unique polyurethane-urea (PUU) fibrous membrane is fabricated by electrospinning. Then PUU was coated by collagen and formed the elasticity hydrogel after immersed in collagen solution. Meanwhile, the cPUU hydrogel exhibited a fibrous microstructure. This cPUU hydrogel had outstanding stretching property with 404 ± 40% elongation at break compared with traditional hydrogels, which satisfied the requirement of urethra. The cPUU hydrogel also supported the adhesion and growth of bladder smooth-muscle cells (BSMCs) in natural state cell morphology. Urethral defects in New Zealand male rabbits were repaired with cPUU seeded with BSMCs in vivo. After three months, more smooth-surface area of reconstructed urethral tissues was observed in the cPUU hydrogel-BMSCs groups compared with that of the control group. The luminal patency and the incidence of complications-including calculus formation, urinary fistula, and urethral-stricture occurrence were significantly lower in the cPUU group compared with that of the control group. Hence, cPUU fibrous hydrogels are promising scaffolds for application in urological tissue engineering.






Similar content being viewed by others
References
Xu YM, Qiao Y, Sa YL, Zhang J, Fu Q, Song LJ. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol. 2009;182:1040–3.
Barbagli G, Kulkarni SB, Fossati N, Larcher A, Sansalone S, Guazzoni G, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol. 2014;192:808–13.
Chapple C, Andrich D, Atala A, Barbagli G, Cavalcanti A, Kulkarni S, et al. SIU/ICUD consultation on urethral strictures: The management of anterior urethral stricture disease using substitution urethroplasty. Urology. 2014;83:S31–47.
Fossati N, Barbagli G, Larcher A, Dell'Oglio P, Sansalone S, Lughezzani G, et al. The surgical learning curve for one-stage anterior urethroplasty: a prospective single-surgeon study. Eur Urol. 2016;69:686–90.
Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12.
Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40.
Farhat WA, Yeger H. Does mechanical stimulation have any role in urinary bladder tissue engineering? World J Urol. 2008;26:301–5.
Beiko DT, Knudsen BE, Watterson JD, Cadieux PA, Reid G, Denstedt JD. Urinary tract biomaterials. J Urol. 2004;171:2438–44.
Lin HK, Madihally SV, Palmer B, Frimberger D, Fung KM, Kropp BP. Biomatrices for bladder reconstruction. Adv drug Deliv Rev. 2015;82-83:47–63.
Song L, Murphy SV, Yang B, Xu Y, Zhang Y, Atala A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng Part B Rev. 2014;20:163–72.
Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS, et al. Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng. 2014;42:1494–507.
Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–6.
Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–33.
Jia W, Tang H, Wu J, Hou X, Chen B, Chen W, et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials. 2015;69:45–55.
Rohman G, Pettit JJ, Isaure F, Cameron NR, Southgate J. Influence of the physical properties of two-dimensional polyester substrates on the growth of normal human urothelial and urinary smooth muscle cells in vitro. Biomaterials. 2007;28:2264–74.
Cui Y, Tan M, Zhu A, Guo M. Non-covalent interaction cooperatively induced stretchy, tough and stimuli-responsive polyurethane-urea supramolecular (PUUS) hydrogels. J Mater Chem B. 2015;3:2834–41.
El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pr. 2013;2013:316–42.
Stier S, Rebers L, Schönhaar V, Hoch E, Borchers K. Advanced formulation of methacryl- and acetyl-modified biomolecules to achieve independent control of swelling and stiffness in printable hydrogels. J Mater Sci. 2019;30:35.
Jun I, Han H-S, Edwards R, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19:745.
Guo T, Yang X, Deng J, Zhu L, Wang B, Hao S. Keratin nanoparticles-coating electrospun PVA nanofibers for potential neural tissue applications. J Mater Sci. 2018;30:9.
Lv X, Guo Q, Han F, Chen C, Ling C, Chen W, et al. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int J Mol Sci. 2016;17:1262.
Jia L, Han F, Wang H, Zhu C, Guo Q, Li J, et al. Polydopamine-assisted surface modification for orthopaedic implants. J Orthop Translation. 2019;17:82–95.
Yang B-Y, Deng G-Y, Zhao R-Z, Dai C-Y, Jiang C-Y, Wang X-J, et al. Porous Se@SiO2 nanosphere-coated catheter accelerates prostatic urethra wound healing by modulating macrophage polarization through reactive oxygen species-NF-κB pathway inhibition. Acta Biomaterialia. 2019;88:392–405.
Shinchi M, Kushibiki T, Mayumi Y, Ito K, Asano T, Ishihara M, et al. Insulin-like growth factor 1 sustained-release collagen on urethral catheter prevents stricture after urethral injury in a rabbit model. Int J Urol. 2019;26:572–7.
Mathur R, Aggarwal G, Satsangi B. A retrospective analysis of delayed complications of urethroplasty at a tertiary care centre. Updates Surg. 2011;63:185–90.
O’Brien FJ. Biomaterials and scaffolds for tissue engineering. Mater Today. 2011;14:88–95.
Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, Zhao W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomaterialia. 2017;50:154–64.
Kotch FW, Raines RT. Self-assembly of synthetic collagen triple helices. Proc Natl Acad Sci USA. 2006;103:3028–33.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Adamowicz J, Juszczak K, Bajek A, Tworkiewicz J, Nowacki M, Marszalek A, et al. Morphological and urodynamic evaluation of urinary bladder wall regeneration: muscles guarantee contraction but not proper function - a rat model research study. Transplant Proc. 2012;44:1429–34.
O'Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev. 2015;21:103–14.
Mousa NA, Abou-Taleb HA, Orabi H. Stem cell applications for pathologies of the urinary bladder. World J Stem Cells. 2015;7:815–22.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9.
Shakhssalim N, Soleimani M, Dehghan MM, Rasouli J, Taghizadeh-Jahed M, Torbati PM, et al. Bladder smooth muscle cells on electrospun poly(epsilon-caprolactone)/poly(l-lactic acid) scaffold promote bladder regeneration in a canine model. Mater Sci Eng C Mater Biol Appl. 2017;75:877–84.
Zakrzewski JL, van den Brink MR, Hubbell JA. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32:786–94.
Zhang H, Jia X, Han F, Zhao J, Zhao Y, Fan Y, et al. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials. 2013;34:2202–12.
Drewa T, Adamowicz J, Sharma A. Tissue engineering for the oncologic urinary bladder. Nat Rev Urol. 2012;9:561–72.
Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–20.
Acknowledgements
This study was supported by the National Key R&D Program of China (2016YFC1100203), National Natural Science Foundation of China (81672213, 31872748), Jiangsu Provincial Clinical Orthopedic Center, and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Authors’ contributions
Weiguo Chen, Fengxuan Han and Bin Li conceived and designed the experiments; Professor Mingyu Guo provided the PUU materials; Chunyang Chen and Chengyuan Wang performed the experiments with the help of Fengxuan Han; Chunyang Chen and Chengyuan Wang analyzed the data; Chengyuan Wang and Fengxuan Han wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., Chen, C., Guo, M. et al. Stretchable collagen-coated polyurethane-urea hydrogel seeded with bladder smooth muscle cells for urethral defect repair in a rabbit model. J Mater Sci: Mater Med 30, 135 (2019). https://doi.org/10.1007/s10856-019-6342-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-019-6342-7