Abstract
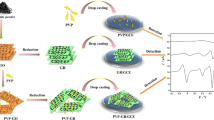
In this paper, an ultra-sensitive glassy carbon electrode modified with β-cyclodextrin and graphene oxide (β-CD-GO/GCE) has been prepared for the detection of biomolecules [ascorbic acid (AA), dopamine (DA) and uric acid (UA)] in aqueous solution at μM level. The three biomolecules (AA, DA and UA) can be simultaneously detected by the modified electrodes, and the electrical signals corresponding to various biomolecules can be identified clearly in cyclic voltammetry and differential pulse voltammetry curves. The simultaneous detection limits of AA, DA, UA were 4.58, 1.56, 1.27 μM, respectively. This ultra-sensitivity should be attributed to the formation of host–guest complexes between β-CD and the biomolecules. Furthermore, the anti-interference experiments indicated the β-CD-GO/GCE exhibited satisfactory selective recognition performance.












Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article.
References
R. Yin, S.Q. Mao, B. Zhao, Z. Chong, Y. Yang, C. Zhao, D. Zhang, H. Huang, J. Gao, Z. Li, Y. Jiao, C. Li, S. Liu, D. Wu, W. Gu, Y.G. Yang, G.L. Xu, H. Wang, Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 135, 10396–10403 (2013)
L.K. Massey, M. Liebman, S.A. Kynast-Gales, Ascorbate increases human oxaluria and kidney stone risk. J. Nutr. 135, 1673–1677 (2005)
D.L. Robinson, V.B. Jill, M. Heien, W.R. Mark, Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 49, 1763–1773 (2003)
A. Krishnan, S. Beena, S.M.A. Shibli, A novel high performance Ti/Ti–W- reinforced polyaniline functionalized Ni–P electrode for high sensitive detection of dopamine from urine sample. Mater. Chem. Phys. 244, 122680 (2020)
S.A. Fathallah-Shaykh, M.T. Cramer, Uric acid and the kidney. Pediatr. Nephrol. 29, 999–1008 (2014)
M. Ali, M.A.U. Khalid, I. Shah, S.W. Kim, Y.S. Kim, J.H. Lim, K.H. Choi, Paper-based selective and quantitative detection of uric acid using citrate-capped Pt nanoparticles (PtNPs) as a colorimetric sensing probe through a simple and remote-based device. New J. Chem. 43, 7636–7645 (2019)
Q. Aemig, D. Patureau, Impact asssesment of a large panel of organic and inorganic micropollutants released by wastewater treatment plants at the scale of France. Water Res. 188, 116524 (2021)
P. Vaudin, C. Auge, N. Just, S. Mhaouty-Kodja, S. Mortaud, D. Pillon, When pharmaceutical drugs become environmental pollutants: potential neural effects and underlying mechanisms. Environ. Res. 205, 112495 (2022)
K. Appan Roychoudhury, JayPatel Antonyfrancis, S. Kumarjha, SuddhasatwaBasu, A decoupler-free simple paper microchip capillary electrophoresis device for simultaneous detection of dopamine, epinephrine and serotonin. RSC Adv. 10, 25487–25495 (2020)
N. Li, J. Guo, B. Liu, Y. Yu, H. Cui, L. Mao, Y. Lin, Determination of monoamine neurotransmitters and their metabolites in a mouse brain microdialysate by coupling high-performance liquid chromatography with gold nanoparticle-initiated chemiluminescence. Anal. Chim. Acta 645, 48–55 (2009)
M.R. Moghadam, S. Dadfarnia, A. Shabani, P. Shahbazikhah, Chemometric-assisted kinetic-spectrophotometric method for simultaneous determination of ascorbic acid, uric acid, and dopamine. Anal. Biochem. 410, 289–295 (2011)
J. Kim, H. Park, J. Ryu, O. Jeon, I.R. Paeng, Competitive enzyme-linked immunosorbent assay for a selective and sensitive determination of dopamine in the presence of ascorbic acid and uric acid. J. Immunoassay Immunochem. 31, 33–44 (2010)
C.L. Sun, C.T. Chang, H.H. Lee, J. Zhou, W.F. Pong, Microwave-assisted synthesis of a core-shell MWCNT/GONR heterostructure for the electrochemical detection of ascorbic acid, dopamine, and uric acid. ACS Nano 5, 7788–7795 (2011)
W. Suginta, P. Khunkaewla, A. Schulte, Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113, 5458–5479 (2013)
P. Reanpang, P. Mool-Am-Kha, J. Upan, J. Jakmunee, A novel flow injection amperometric sensor based on carbon black and graphene oxide modified screen-printed carbon electrode for highly sensitive determination of uric acid. Talanta 232, 122493 (2021)
M. Mazloum-Ardakani, H. Beitollahi, M.K. Amini, F. Mirkhalaf, M. Abdollahi-Alibeik, New strategy for simultaneous and selective voltammetric determination of norepinephrine, acetaminophen and folic acid using ZrO2 nanoparticles-modified carbon paste electrode. Sens. Actuators, B Chem. 151, 243–249 (2010)
M. Liu, Q. Chen, C. Lai, Y. Zhang, J. Deng, H. Li, S. Yao, A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe(3)O(4)@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 48, 75–81 (2013)
X. Lu, S. Li, W. Guo, F. Zhang, F. Qu, A covalent organic polymer–TiO2/Ti3C2 heterostructure as nonenzymatic biosensor for voltammetric detection of dopamine and uric acid. Microchim. Acta 188, 95 (2021)
L. Yang, N. Huang, Q. Lu, M. Liu, H. Li, Y. Zhang, S. Yao, A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 903, 69–80 (2016)
B. Habibi, M. Jahanbakhshi, M.H. Pournaghi-Azar, Simultaneous determination of acetaminophen and dopamine using SWCNT modified carbon–ceramic electrode by differential pulse voltammetry. Electrochim. Acta 56, 2888–2894 (2011)
Y. Zhang, Z. Chen, L. Yao, X. Wang, Q.M. Fu, Z.D. Lin, S.G. Wang, Study of ion permeation theough the graphene oxide/polyether sulfone membranes. ChemElectroChem 7, 493–499 (2020)
A. Abellán-Llobregat, L. Vidal, R. Rodríguez-Amaro, Á. Berenguer-Murcia, A. Canals, E. Morallón, Au-IDA microelectrodes modified with Au-doped graphene oxide for the simultaneous determination of uric acid and ascorbic acid in urine samples. Electrochim. Acta 227, 275–284 (2016)
H.T.N. Le, H.K. Jeong, Cyclodextrin-graphite oxide-carbon nanotube composites for electrochemical supramolecular recognition. Electrochim. Acta 232, 7–12 (2017)
J. Szejtli, Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
W. Tang, S.C. Ng, Facile synthesis of mono-6-amino-6-deoxy-α-, β-, γ-cyclodextrin hydrochlorides for molecular recognition, chiral separation and drug delivery. Nat. Protoc. 3, 691–697 (2008)
Y. Liu, Y. Chen, Cooperative binding and multiple recognition by bridged Bis(β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 39, 681–691 (2006)
S.P. Kusumocahyo, K. Sumaru, T. Kanamori, T. Iwatsubo, T. Shinbo, Synthesis and characterization of an ultrathin polyion complex membrane containing β-cyclodextrin for separation of organic isomers. J. Membr. Sci. 230, 171–174 (2004)
G. Zhu, X. Zhang, P. Gai, X. Zhang, J. Chen, β-Cyclodextrin non-covalently functionalized single-walled carbon nanotubes bridged by 3,4,9,10-perylene tetracarboxylic acid for ultrasensitive electrochemical sensing of 9-anthracenecarboxylic acid. Nanoscale 4, 5703–5709 (2012)
R.P. Bell, R.R. Robinson, The ionization constants of some acids in dioxan+water mixtures. Trans. Faraday Soc. 57, 965–970 (1961)
T. Ishimutsu, S. Hirose, H. Sakurai, Microscopic acid dissociation constants of 3,4-dihydroxyphenethylamine (Dopamine). Chem. Pharm. Bull. 26, 74–78 (1978)
A. Albert, D.J. Brown, Purine studies part I stability to acid and alkali solubility ionization comparison with pteridines. J. Chem. Soc. (1954). https://doi.org/10.1039/jr9540002060
S.P. Zhang, B. Liu, C.Y. Li, W. Chen, Z.J. Yao, D.T. Yao, R.B. Yu, H.O. Song, S., Enhanced dispersibility and thermal stability of β-cyclodextrin functionalized graphene. Chin. Chem. Lett. 25, 355–358 (2014)
J. Gao, S. Zhang, M. Liu, Y. Tai, X. Song, Y. Qian, H. Song, Synergistic combination of cyclodextrin edge-functionalized graphene and multiwall carbon nanotubes as conductive bridges toward enhanced sensing response of supramolecular recognition. Electrochim. Acta 187, 364–374 (2016)
B. Kaur, T. Pandiyan, B. Satpati, R. Srivastava, Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf., B 111, 97–106 (2013)
T. Lin, K.G. Zhou, Y.H. Zhang, H.X. Wang, X.D. Wang, Y.F. Guo, H.L. Zhang, Nanomolar detection of dopamine in the presence of ascorbic acid at β-cyclodextrin/graphene nanocomposite platform. Electrochem. Commun. 12, 557–560 (2010)
D. Jia, J. Dai, H. Yuan, L. Lei, D. Xiao, Selective detection of dopamine in the presence of uric acid using a gold nanoparticles-poly(luminol) hybrid film and multi-walled carbon nanotubes with incorporated β-cyclodextrin modified glassy carbon electrode. Talanta 85, 2344–2351 (2011)
C. Yin, S. Wang, Y.J. Zhang, Z. Chen, Z.D. Lin, P. Fu, L. Yao, Correlation between the pore resistance and water flux of the cellulose acetate membrane. Environ. Sci.: Water Res. Technol. 3, 1037–1041 (2017)
Q. Huang, Q. Luo, Z. Chen, L. Yao, P. Fu, Z.D. Lin, The effect of electrolyte concentration on electrochemical impedance for evaluating polysulfone membranes. Environ. Sci.: Water Res. Technol. 4, 1145–1151 (2018)
A.A. Ensafi, B. Rezaei, S.Z.M. Zare, M. Taei, Simultaneous determination of ascorbic acid, epinephrine, and uric acid by differential pulse voltammetry using poly(3,3′-bis[N, N-bis(carboxymethyl)aminomethyl]-o-cresolsulfonephthalein) modified glassy carbon electrode. Sens. Actuators, B Chem. 150, 321–329 (2010)
N. Murugan, R. Jerome, M. Preethika, A. Sundaramurthy, A.K. Sundramoorthy, 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid - sciencedirect. J. Mater. Sci. Technol. 72, 122–131 (2021)
P. Wu, Y. Huang, X. Zhao, D. Lin, L. Xie, Z. Li, Z. Zhu, H. Zhao, M. Lan, MnFe2O4/MoS2 nanocomposite as Oxidase-like for electrochemical simultaneous detection of ascorbic acid, dopamine and uric acid. Microchem. J. 181, 107780 (2022)
H. Wang, A. Xie, S. Li, J. Wang, K. Chen, Z. Su, N. Song, S. Luo, Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. Anal. Chim. Acta 1211, 339907 (2022)
S. Krishnan, L. Tong, S. Liu, R. Xing, A mesoporous silver-doped TiO2-SnO2 nanocomposite on g-C3N4 nanosheets and decorated with hierarchical core-shell metal-organic framework for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 187, 82 (2020)
H. Huang, Y. Yue, Z. Chen, Y. Chen, S. WU, J. Liao, S. Liu, H.R. Wen, Electrochemical sensor based on a nanocomposite prepared from TmPO4 and graphene oxide for simultaneous voltammetric detection of ascorbic acid, dopamine and uric acid. Microchim. Acta (2019). https://doi.org/10.1007/s00604-019-3299-7
D.Y. Zhao, D.W. Fan, J.P. Wang, C.X. Xu, Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microshimica Acta 182, 1345–1352 (2015)
E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 101, 19–28 (1979)
S.E. Bohndiek, M.I. Kettunen, D.E. Hu, B.W. Kennedy, J. Boren, F.A. Gallagher, K.M. Brindle, Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: vitamin C as a probe for imaging redox status in vivo. J. Am. Chem. Soc. 133, 11795–11801 (2011)
G. Alarcón-Angeles, B. Pérez-López, M. Palomar-Pardave, M.T. Ramírez-Silva, S. Alegret, A. Merkoçi, Enhanced host–guest electrochemical recognition of dopamine using cyclodextrin in the presence of carbon nanotubes. Carbon 46, 898–906 (2008)
A. Abbaspour, A. Noori, A cyclodextrin host-guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosensors andBioelectronics 26, 4674–4680 (2011)
A.A. Ensafi, M. Taei, T. Khayamian, Simultaneous determination of ascorbic acid, epinephrine, and uric acid by differential pulse voltammetry using poly(p-xylenolsulfonephthalein) modified glassy carbon electrode. Colloids Surf., B 79, 480–487 (2010)
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. QG: Material preparation was performed. QG, SL and XM: Data collection and analysis were performed. QG: The first draft of the manuscript was written and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Q., Liu, S., Men, X. et al. Sensitive determination of ascorbic acid, dopamine and uric acid by glassy carbon electrodes modified with β-cyclodextrin and graphene oxide. J Mater Sci: Mater Electron 33, 23566–23579 (2022). https://doi.org/10.1007/s10854-022-09116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09116-6