Abstract
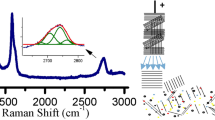
The sulfur-assisted exfoliation of graphite was done in a planetary ball milling. A detailed study with varying milling times, of the Sulfur/exfoliated composite, is presented. Raman spectroscopy was applied to study the carbon material present in the composite. Various parameters like crystallite size (La = 33), band ratios (I2D/IG = 0.55, ID/IG = 1.12), FWHM, etc., emphasises the successful exfoliation of graphite into few-layer exfoliated graphite material. The deconvolution of the d002 peak into two components of the X-ray diffraction pattern solidifies the sulfur impregnation and intercalation in the composite with a high degree of the amorphous layered carbon structure. The XRD analysis is in line with the Raman spectroscopy findings (La = 37). The sulfur present in the composite was analysed with the DSC, where various aspects of sulfur and sulfur-EG interaction were discussed. Various thermodynamic quantities (∆H, ΔCp) were extracted from this study to fully understand the sulfur-EG interface. Three to four-layer graphene has been produced with a high amount of sulfur in it which makes it a suitable option for its application in the conversion type cathode of lithium-ion battery. The milling time is also a key factor that determines the quality of layered material and so the composite. This composite material is cost-effective and environment-friendly. We have used it as a cathode for lithium–sulfur battery and found an initial capacity of 1550 mAh/g which is discussed with its electrochemical performance.









Similar content being viewed by others
Data availability
The authors declare that data supporting the findings of this study are available within the article.
References
A.G. Olabi, M.A. Abdelkareem, T. Wilberforce, E.T. Sayed, Application of graphene in energy storage device—a review. Renew. Sustain. Energy Rev. 135, 110026 (2021). https://doi.org/10.1016/J.RSER.2020.110026
Y. Cao, K. Sharma, A.A. Rajhi, S. Alamri, A.E. Anqi, A.S. El-Shafay, A.A. Aly, B.F. Felemban, S. Rashidi, M. Derakhshandeh, Boron-carbide nanosheets: promising anodes for Ca-ion batteries. J. Electroanal. Chem. 910, 115929 (2022). https://doi.org/10.1016/J.JELECHEM.2021.115929
A. Kumar, K. Sharma, A.R. Dixit, A review of the mechanical and thermal properties of graphene and its hybrid polymer nanocomposites for structural applications. J. Mater. Sci. 54, 5992–6026 (2019). https://doi.org/10.1007/S10853-018-03244-3/TABLES/4
N. Sharma, V. Sharma, Y. Jain, M. Kumari, R. Gupta, S.K. Sharma, K. Sachdev, Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 376, 1700006 (2017). https://doi.org/10.1002/masy.201700006
P.K. Singh, K. Sharma, P.K. Singh, A low cost, bulk synthesis of the thermally reduced graphene oxide in an aqueous solution of sulphuric acid & hydrogen peroxide via electrochemical method. Inorg. Chem. Commun. 140, 109378 (2022). https://doi.org/10.1016/J.INOCHE.2022.109378
A. Kumar, K. Sharma, A.R. Dixit, Carbon nanotube- and graphene-reinforced multiphase polymeric composites: review on their properties and applications. J. Mater. Sci. 55, 2682–2724 (2020). https://doi.org/10.1007/S10853-019-04196-Y/FIGURES/21
G. Kucinskis, G. Bajars, J. Kleperis, Graphene in lithium ion battery cathode materials: a review. J. Power Sources 240, 66–79 (2013). https://doi.org/10.1016/J.JPOWSOUR.2013.03.160
X. Zhang, Research progress of high performance cathode materials for lithium-sulfur batteries. IOP Conf. Ser. Earth Environ. Sci. 781, 042051 (2021). https://doi.org/10.1088/1755-1315/781/4/042051
A. Eftekhari, D.W. Kim, Cathode materials for lithium-sulfur batteries: a practical perspective. J. Mater. Chem. A 5, 17734–17776 (2017). https://doi.org/10.1039/c7ta00799j
S.R. Chen, Y.P. Zhai, G.L. Xu, Y.X. Jiang, D.Y. Zhao, J.T. Li, L. Huang, S.G. Sun, Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium–sulfur battery. Electrochim. Acta. 56, 9549–9555 (2011). https://doi.org/10.1016/J.ELECTACTA.2011.03.005
Y. Lu, S. Zhang, J. Yin, C. Bai, J. Zhang, Y. Li, Y. Yang, Z. Ge, M. Zhang, L. Wei, M. Ma, Y. Ma, Y. Chen, Mesoporous activated carbon materials with ultrahigh mesopore volume and effective specific surface area for high performance supercapacitors. Carbon N. Y. 124, 64–71 (2017). https://doi.org/10.1016/J.CARBON.2017.08.044
L. Serrano-Luján, S. Víctor-Román, C. Toledo, O. Sanahuja-Parejo, A.E. Mansour, J. Abad, A. Amassian, A.M. Benito, W.K. Maser, A. Urbina, Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl. Sci. 1, 1–12 (2019). https://doi.org/10.1007/S42452-019-0193-1/FIGURES/5
S. Yousef, A. Mohamed, M. Tatariants, Mass production of graphene nanosheets by multi-roll milling technique. Tribol. Int. 121, 54–63 (2018). https://doi.org/10.1016/J.TRIBOINT.2018.01.040
T. Lin, Y. Tang, Y. Wang, H. Bi, Z. Liu, F. Huang, X. Xie, M. Jiang, Scotch-tape-like exfoliation of graphite assisted with elemental sulfur and graphene–sulfur composites for high-performance lithium-sulfur batteries. Energy Environ. Sci. 6, 1283–1290 (2013). https://doi.org/10.1039/C3EE24324A
S. Soni, A. Sodhiya, A.K. Singh, S. Patel, R. Kumar, The exfoliation of graphite and production of graphene-sulfur composite via ball milling for lithium sulfur-batteries. 3Rd Int. Conf. Condens. Matter Appl. Phys. 2220, 140047 (2020). https://doi.org/10.1063/5.0001184
H.C. Schniepp, J.-L. Li, M.J. McAllister, H. Sai, M. Herrera-Alonso, D.H. Adamson, R.K. Prud’homme, R. Car, D.A. Saville, I.A. Aksay, Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 110, 8535–8539 (2006). https://doi.org/10.1021/jp060936f
F. Erdemir, Study on particle size and X-ray peak area ratios in high energy ball milling and optimization of the milling parameters using response surface method. Measurement 112, 53–60 (2017). https://doi.org/10.1016/J.MEASUREMENT.2017.08.021
A.E. Del Rio-Castillo, C. Merino, E. Díez-Barra, E. Vázquez, Selective suspension of single layer graphene mechanochemically exfoliated from carbon nanofibres. Nano Res. 77(7), 963–972 (2014). https://doi.org/10.1007/S12274-014-0457-4
E.O. Hall, The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Soc. Sect. B 64, 747 (1951). https://doi.org/10.1088/0370-1301/64/9/303
G.I. Taylor, The mechanism of plastic deformation of crystals. Part I.—Theoretical. Proc. R. Soc. London. Ser. A 145, 362–387 (1934). https://doi.org/10.1098/RSPA.1934.0106
S. Soni, A. Sodhiya, S. Patel, S. Patel, R. Kumar, Finding a facile way to exfoliate graphite electrochemically for energy storage device application. MRS Adv. 6, 594–598 (2021). https://doi.org/10.1557/s43580-021-00130-0
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prud’homme, I.A. Aksay, R. Car, Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8, 36–41 (2008). https://doi.org/10.1021/nl071822y
T. Lin, Y. Tang, Y. Wang, H. Bi, Z. Liu, F. Huang, X. Xie, M. Jiang, Scotch-tape-like exfoliation of graphite assisted with elemental sulfur and graphene-sulfur composites for high-performance lithium-sulfur batteries. Energy Environ. Sci. 6, 1283–1290 (2013). https://doi.org/10.1039/c3ee24324a
L.M. Malard, M.A. Pimenta, G. Dresselhaus, M.S. Dresselhaus, Raman spectroscopy in graphene. Phys. Rep. 473, 51–87 (2009). https://doi.org/10.1016/j.physrep.2009.02.003
A.C. Ferrari, Raman spectroscopy of graphene and graphite: disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47–57 (2007). https://doi.org/10.1016/j.ssc.2007.03.052
F. Tuinstra, J.L. Koenig, Raman spectrum of graphite. J. Chem. Phys. (1970). https://doi.org/10.1063/1.1674108
L.G. Caņado, K. Takai, T. Enoki, M. Endo, Y.A. Kim, H. Mizusaki, A. Jorio, L.N. Coelho, R. Magalhães-Paniago, M.A. Pimenta, General equation for the determination of the crystallite size la of nanographite by Raman spectroscopy. Appl. Phys. Lett. 88, 163106 (2006). https://doi.org/10.1063/1.2196057
Y. Bleu, F. Bourquard, A. Sophie, L. Vincent, F. Garrelie, C. Donnet, Raman study of the substrate influence on graphene synthesis using a solid carbon source via rapid thermal annealing. J. Raman Spectrosc. 50, 1630–1641 (2019). https://doi.org/10.1002/jrs.5683
G. George, S.B. Sisupal, T. Tomy, A. Kumaran, P. Vadivelu, V. Suvekbala, S. Sivaram, L. Ragupathy, Facile, environmentally benign and scalable approach to produce pristine few layers graphene suitable for preparing biocompatible polymer nanocomposites. Sci. Rep. 8, 1–15 (2018). https://doi.org/10.1038/s41598-018-28560-1
I.A. Kinloch, R.A.W. Dryfe, Two-step electrochemical intercalation and oxidation of graphite for the mass production of graphene oxide. J. Am. Chem. Soc. (2017). https://doi.org/10.1021/jacs.7b08515
V. León, A.M. Rodriguez, P. Prieto, M. Prato, E. Vázquez, Exfoliation of graphite with trriazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 8, 563–571 (2014). https://doi.org/10.1021/nn405148t
J. Hannauer, J. Scheers, J. Fullenwarth, B. Fraisse, The quest for polysulfides in lithium–sulfur battery electrolytes: an operando confocal raman spectroscopy study. ChemPhysChem 16, 2755–2759 (2015). https://doi.org/10.1002/cphc.201500448
A. Eckmann, A. Felten, A. Mishchenko, L. Britnell, R. Krupke, K.S. Novoselov, C. Casiraghi, Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 12, 3925–3930 (2012). https://doi.org/10.1021/nl300901a
Y. Quan, Q. Liu, S. Zhang, S. Zhang, Comparison of the morphology, chemical composition and microstructure of cryptocrystalline graphite and carbon black. Appl. Surf. Sci. 445, 335–341 (2018). https://doi.org/10.1016/J.APSUSC.2018.03.182
L. Alexander, H.P. Klug, Determination of crystallite size with the X-ray spectrometer. J. Appl. Phys. 21, 137 (2004). https://doi.org/10.1063/1.1699612
P. Scherrer, Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen, Nachrichten von Der Gesellschaft Der Wissenschaften Zu Göttingen, Math. Klasse. 1918 (n.d.) 98–100. https://eudml.org/doc/59018#.YujJYL5iAMs.mendeley. Accessed 2 Aug 2022
J.I. Langford, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11, 102–113 (1978). https://doi.org/10.1107/S0021889878012844
V. Uvarov, I. Popov, Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 85, 111–123 (2013). https://doi.org/10.1016/J.MATCHAR.2013.09.002
C. Heo, H.G. Moon, C.S. Yoon, J.H. Chang, ABS nanocomposite films based on functionalized-graphene sheets. J. Appl. Polym. Sci. 124, 4663–4670 (2012). https://doi.org/10.1002/APP.35404
C. Yao, Y. Sun, K. Zhao, T. Wu, A. Mauger, C.M. Julien, L. Cong, J. Liu, H. Xie, L. Sun, Self-assembled layer-by-layer partially reduced graphene oxide–sulfur composites as lithium–sulfur battery cathodes. RSC Adv. 8, 3443–3452 (2018). https://doi.org/10.1039/C7RA12194F
X. He, L. Wang, W. Pu, J. Ren, W. Wu, C. Jiang, C. Wan, Thermal analysis of sulfurization of polyacrylo- nitrile with elemental sulfur. J. Therm. Anal. Calorim. 94, 151–155 (2008)
C. Lv, H. Wu, W. Lin, J.B. Illerup, A.P. Karcz, S. Ye, A.J. Damø, Characterization of elemental sulfur in chalcopyrite leach residues using simultaneous thermal analysis. Hydrometallurgy 188, 22–30 (2019). https://doi.org/10.1016/J.HYDROMET.2019.05.020
X.M. He, L. Wang, W.H. Pu, J.G. Ren, W. Wu, C.Y. Jiang, C.R. Wan, Thermal analysis of sulfurization of polyacrylonitrile with elemental sulfur. J. Therm. Anal. Calorim. 94, 151–155 (2008). https://doi.org/10.1007/S10973-008-9008-0
G. Carotenuto, V. Romeo, S. De Nicola, L. Nicolais, Graphite nanoplatelet chemical cross-linking by elemental sulfur. Nanoscale Res. Lett. 8, 94 (2013). https://doi.org/10.1186/1556-276X-8-94
Acknowledgements
The author wants to acknowledge the facilities provided by the Sophisticated Instrument Center at Dr. Harisingh Gour Vishwavidyalaya, Sagar for the characterization and the National Center for Photovoltaic Research and Education for its support in electrochemical measurements under the Photovoltaic Users Mentorship Programme (PUMP).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SS: conceptualization of the work, the acquisition, analysis, and interpretation of data. Original draft and correspondence. RK: conceptualization and approved the version to be published. AS, SP: investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soni, S., Kumar, R., Sodhiya, A. et al. From defects to charge conjugation: a combined approach to analyze sulfur-assisted exfoliation of graphite for its application in a lithium–sulfur battery cathode. J Mater Sci: Mater Electron 33, 23375–23389 (2022). https://doi.org/10.1007/s10854-022-09099-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09099-4