Abstract
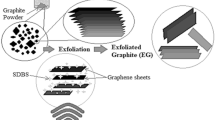
The process of making single-layer graphene is encumbered by price and includes hazardous chemicals. The graphite layers, which are weakly bonded by van der Waals force, can be Segregated or exfoliated into less than three layers via mechanical or chemical exfoliation. The electrochemical exfoliation of graphite is eco-friendly, affordable, and straightforward. Various types of electrolytes can be used to perform this process, but here we have taken 1 molar ammonium sulfate solution in deionized water which incorporates a trace of sulfur into the graphene layers as well. This sulfur-doped material forms a composite type structure (EG/sulfur) where the amount of sulfur can be increased via further processing and the sulfur absorption makes this exfoliated graphite different from the chemically exfoliated graphite. We have performed an electrochemical exfoliation of graphite in ammonium sulfate solution in different voltage conditions and 12 V was found to be an ideal voltage during the exfoliation and to produce two- to three-layer graphene.
Graphic abstract




Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
R. Muñoz, C. Gómez-Aleixandre, Review of CVD synthesis of graphene. Chem. Vap. Depos. 19, 297–322 (2013). https://doi.org/10.1002/cvde.201300051
D. Van Thanh, L.J. Li, C.W. Chu, P.J. Yen, K.H. Wei, Plasma-assisted electrochemical exfoliation of graphite for rapid production of graphene sheets. RSC Adv. 4, 6946–6949 (2014). https://doi.org/10.1039/c3ra46807k
A.T. Najafabadi, E. Gyenge, Synergistic production of graphene microsheets by simultaneous anodic and cathodic electro-exfoliation of graphitic electrodes in aprotic ionic liquids. Carbon N. Y. 84, 449–459 (2015). https://doi.org/10.1016/j.carbon.2014.12.041
A.T. Najafabadi, E. Gyenge, High-yield graphene production by electrochemical exfoliation of graphite: Novel ionic liquid (IL)-acetonitrile electrolyte with low IL content. Carbon N. Y. 71, 58–69 (2014). https://doi.org/10.1016/j.carbon.2014.01.012
A.T. Najafabadi, N. Ng, E. Gyenge, Electrochemically exfoliated graphene anodes with enhanced biocurrent production in single-chamber air-breathing microbial fuel cells. Biosens. Bioelectron. 81, 103–110 (2016). https://doi.org/10.1016/j.bios.2016.02.054
N. Sharma, V. Sharma, Y. Jain, M. Kumari, R. Gupta, S.K. Sharma, K. Sachdev, Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 376, 1700006 (2017). https://doi.org/10.1002/masy.201700006
A.C. Ferrari, Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47–57 (2007). https://doi.org/10.1016/j.ssc.2007.03.052
G. Radhika, R. Subadevi, K. Krishnaveni, W.R. Liu, M. Sivakumar, Synthesis and electrochemical performance of PEG-MnO2-Sulfur composites cathode materials for lithium-sulfur batteries. J. Nanosci. Nanotechnol. 18, 127–131 (2018). https://doi.org/10.1166/jnn.2018.14568
T. Lin, Y. Tang, Y. Wang, H. Bi, Z. Liu, F. Huang, X. Xie, M. Jiang, Scotch-tape-like exfoliation of graphite assisted with elemental sulfur and graphene-sulfur composites for high-performance lithium-sulfur batteries. Energy Environ. Sci. 6, 1283–1290 (2013). https://doi.org/10.1039/c3ee24324a
L.M. Malard, M.A. Pimenta, G. Dresselhaus, M.S. Dresselhaus, Raman spectroscopy in graphene. Phys. Rep. 473, 51–87 (2009). https://doi.org/10.1016/j.physrep.2009.02.003
A. Eckmann, A. Felten, A. Mishchenko, L. Britnell, R. Krupke, K.S. Novoselov, C. Casiraghi, Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 12, 3925–3930 (2012). https://doi.org/10.1021/nl300901a
G. George, S.B. Sisupal, T. Tomy, A. Kumaran, P. Vadivelu, V. Suvekbala, S. Sivaram, L. Ragupathy, Facile, environmentally benign and scalable approach to produce pristine few layers graphene suitable for preparing biocompatible polymer nanocomposites. Sci. Rep. 8, 11228 (2018). https://doi.org/10.1038/s41598-018-28560-1
C. Casiraghi, A. Hartschuh, H. Qian, S. Pliscanec, C. Georgia, A. Fasoli, K.S. Novoselov, D.M. Basko, A.C. Ferrari, Raman spectroscopy of graphene edges. Nano Lett. 9, 1433–1441 (2009). https://doi.org/10.1021/nl8032697
S. Evers, L.F. Nazar, Graphene-enveloped sulfur in a one pot reaction: A cathode with good coulombic efficiency and high practical sulfur content. Chem. Commun. 48, 1233–1235 (2012). https://doi.org/10.1039/c2cc16726c
Acknowledgments
The authors thankfully acknowledge DST-PURSE (PHASE-II) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Soni, S., Sodhiya, A., Patel, S. et al. Finding a facile way to exfoliate graphite electrochemically for energy storage device application. MRS Advances 6, 594–598 (2021). https://doi.org/10.1557/s43580-021-00130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-021-00130-0