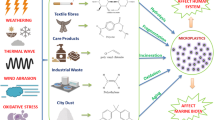
Abstract
Over the last century, accumulation of microplastic has emerged as a greater threat to the environment, plants, microorganisms and even human beings. Microplastics can be intentionally produced for industries such as cosmetics, or they may be unintentionally generated from degradation of bulk plastic debris. Furthermore, mismanagement of plastic waste is a major source of microplastics. When ingested, microplastics can alter several physical, chemical and biological processes in living organisms. Thus, their toxicity silently spreads its roots into the biosphere. Unfortunately, current strategies for the elimination of microplastics are not sufficient for their complete removal and degradation. Therefore, the adoption of green innovative technologies is the first step toward a microplastic-free environment. However, advances for its effective degradation and elimination are hindered by our limited understanding. This literature study investigates microplastic comprehensively, covering their sources, fate, ecological impacts and their effects on biological processes. It includes an analysis of microplastics in Indian rivers, explores methods for its eradication and degradation, emphasizes plastic recycling and offers future recommendations to pave way toward achieving a microplastic-free environment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plastic has progressed from essentially non-existent to a pervasive and important aspect of contemporary life throughout the last century. It has been estimated that plastic manufacture has significantly expanded over the last few decades. According to a recent report, global plastic production had soared to 360 million tons in 2018 [1]. Asia stands as the leading producer, accounting for 50% of the total, followed by North American Free Trade Agreement (NAFTA) and European countries [1]. Plastic can be easily produced via facile chemical or physical reactions. The two basic procedures are polymerization and polycondensation, which fundamentally change the primary components into polymer chains, giving rise to different compositions of plastic. These are irreversible processes, and once they are disposed of, plastics make way into various biological, chemical and environmental systems. Supplanted usage of plastics in our day-to-day lives is due to their high durability, inertness and comparative low cost. Due to their extreme adaptability, plastics have become an essential component of the medical industry. Despite the substantial global plastic waste production, only a small fraction undergoes recycling. Most of this generated plastic waste is dumped or burned in the landfills [2]. The disposal of plastic into landfills ultimately causes detrimental effects on the environment. The situation is worse as their natural disintegration process becomes more difficult, subsequently accumulating plastic in practically every form of environment [3].
The escalating issue of plastic pollution is prominently evident by the pervasive occurrence of plastic waste in terrestrial and aquatic ecosystems. Extensive scientific investigations have been conducted on developing analytical methodologies, exploring the sources and abundance, fate and degradation of plastics in the environment, as well as risks to natural environments, wildlife and even human health. However, the bulky macro-sized debris of plastic exert limited direct influence on both biotic and abiotic components of the environment. It is, rather, at the smaller—the micro- and nanosized level, that these pollutants possess the potential to permeate the biosphere.
In 2004, Thompson et al. [4] introduced the world with a new type of plastic pollutant, termed “Microplastic.” These are plastics having an effective size < 5 mm. Currently, with the recent advancement in studies on plastic-derived pollution in our environment, plastic trash has been generically classified into four size classes: nanoplastics (NPs), microplastics (MPs), mesoplastics and macroplastics (MaPs) [5]. NPs comprise plastic size less than 100 nm, MPs range between 0.1 and 5 mm, mesoplastics range between 5 and 25 mm and MaPs comprise plastic size greater than 25 mm [5]. Vast accumulation of MaPs, originating from domestic, commercial and industrial waste, proliferate on land and water, causing an intoxicating impact on all land, marine, and freshwater life forms. For instance, it has been reported that suffocation due to consumption of plastic bags can lead to death in cattle [6]. The accumulated MaPs are further broken down into MPs by various physical, chemical and biological processes. These MPs can penetrate aquatic and terrestrial environments and eventually find their way into the human body.
Intriguingly, recent studies have determined that the detrimental effect of the total plastic litter generated on our planet is a contribution of microplastics and nanoplastics rather than MaPs [7]. According to environmental surveys, the accumulation of MaPs remains steady or decreases, whereas that of MPs increases [8]. Various studies have revealed MPs can be derived from microbeads and fibers, which is depicted in Fig. 1 [9]. Similar studies were carried out on the Juhu beach (Mumbai, India), where plastic litter was assessed to significantly accumulate MPs [10]. Other worldwide studies have also concluded that MPs can be present in the biosphere, creating toxic consequences among life forms. Similarly, it has also been reported that the contamination of MPs in aquatic ecosystems promotes biofouling, which hampers their buoyancy [11]. Thus, it must be emphasized, despite the presence of four different plastic pollutants in our ecosystem, MPs pose the largest threat as compared to macro-, meso- and nanoplastics. Therefore, due to the numerous concerns related to MP contamination, the uncontrolled discharge in the biosphere must be regulated thoroughly. However, a lack of understanding and analytical approaches for MP synthesis, analysis and removal impedes further growth toward an MP-free environment.

Reproduced with permission from reference [9]. Copyright © 2018 Elsevier Ltd. All rights reserved
(A) and (B) Primary MPs derived from personal care products, (C) and (D) secondary MPs derived from synthetic textiles and fibers.
This literature study aims to address the novel aspects of microplastics, demonstrating their diverse sources, fate and ecological consequences, with a particular emphasis on their potential impacts on biological processes across a wide variety of species. This review presents a novel investigation into an unexplored area of concern, specifically focusing on the prevalence of MP concentrations in rivers located within developing countries. This aspect has not been previously examined in recent studies, making this review the first to discuss this particular scenario in Indian rivers. Furthermore, this literature study rigorously consolidates advanced approaches related to the eradication and degradation of MPs, emphasizing recent advancements in thermal degradation, photocatalytic degradation, and biodegradation techniques tailored to the characteristics of MPs. Moreover, in the pursuit to optimize treatment steps within wastewater treatment plants, an array of techniques specific to effective MP removal have been succinctly elucidated. Additionally, it provides a unique perspective on the significance of plastic recycling. Finally, this study highlights the challenges associated with MP degradation and removal methods and offers novel insights and future recommendations to facilitate the attainment of MP-free environment. Such a study will aid in creating a better understanding of the current MPs pollutants as well as solutions to address them.
Sources and fate
Plastic has permeated nearly every aspect of modern life since the industrial revolution. Their diverse applications are credited to its low cost, durability, abundance, lightweight, and malleability. Different grades of plastics are incorporated in consumer and industrial plastic products. Recent estimations have predicted that annual plastic waste could climb up to 53 million metric tons, gradually exceeding our ability to halt plastic pollutants leaking into various ecosystems [12]. Some of the major demanded plastics are polyvinyl chloride (PVC), polyethylene (PE), polyacrylonitrile (PAN), polyethylene terephthalate (PET), polypropylene (PP), polyamide (PA), polyurethane (PU) and polystyrene (PS). Applications of these plastics range from basic life necessities such as a mere plastic toothbrush and containers to major industrial requirements such are tires and synthetic fibers. In addition to these polymers, recent studies were also able to raise concerns regarding the toxic effects of increasing usage of poly lactic acid (PLA) [13]. PLA has emerged as a potential substitute for petroleum-based plastics. However, recent research highlights that PLA is compostable rather than biodegradable in natural environments, resulting in the generation of MPs [14, 15]. Subsequently, the progressive accumulation, as a resultant of the linear flow of plastics, impacts the environment in many folds. It is important to consider all of the variables that can affect plastic particle's characteristics over the course of its life, from manufacture to consumption. The seeping of MPs into various ecosystems can occur through two ways: primary and secondary sources.
Primary sources of MPs are industrially produced as such and are directly released in the form of small particulates [2]. Most often consumer and industrial products such as cosmetics, hair care and skin care products, scrubbing agents in toiletries, deodorants, inks, and paints are major primary sources of MPs (Fig. 2). Personal care items containing microbeads, such as toothpaste, shower gels, and facial cleansers, are some of the major primary contributors [16]. MPs derived from primary sources are called primary MPs [17]. So, these kinds of MPs are intentionally produced and designed as such for applications such as plastic microspheres, synthetic textile and personal care products.
MaPs can undergo fragmentation, aging, or weathering to produce smaller bits of plastics, known as microplastics (MPs). This leads to generation of secondary source of MPs. So, secondary MPs are derived from unintentional chemical, physical or biological strain on MaPs, leading to fragmentation and production of MPs, which can potentially enter different aquatic and terrestrial ecosystems [5]. Generally, weathering and aging are caused by the transformation and breakdown of the polymers through the combination of a variety of complex processes [5]. Significant contribution of secondary MPs is seen from the fragmentation of plastic debris such as synthetic fibers, tires and coatings [5]. These MPs which may leach into water bodies can be mistaken as food by worms and fishes and make their way up into the food chain, subsequently contaminating human food [18, 19].
In an attempt to qualitatively and quantitatively understand the degradative fate and persistence of microplastic fibers (MPFs), such as PET, PAN and PA, a group of researchers have explored photolytic degradation of MPFs on UV exposure for a period of 10 months [20]. It was observed that PET and PA exhibited significant fragmentation and surface changes, while PAN did not undergo any drastic changes (Fig. 3). However, their study suggests that more elaborative analysis needs to be performed for assessing the existence, impact and consequences of chemical additive leaching from these MPFs. Besides the direct effect of MPs, chemical additives in plastics also need to be thoroughly investigated. Chemical additives have been used as UV stabilizers, softeners and flame retardants for the production of plastics with enhanced physical properties [5]. Various harmful plastic chemical additives, such as bisphenols, phthalates, and brominated flames retardants, are reported to leach from the polymer surface into its immediate surroundings under the influence of environmental parameters such as extent of exposure to UV light and temperature [21]. Thus, the combined leaching of MPs and plastic chemical additives is a crucial aspect of plastic-derived pollution.

Reproduced with permission from reference [20]. Copyright © 2020 The Author(s). Published by Elsevier Ltd
An environmentally relevant study of fragmentation of common MPFs (PET, PA and PAN) from synthetic textiles under the exposure of UV light; UV ultraviolet.
The common mode of MPs entry in the terrestrial system is through soil via human activities such as agricultural irrigation, plastics mulching, incomplete MPs removal from sludge extraction methods and atmospheric deposition of fibrous MPs [22]. This can hamper various soil properties such as porosity and aggregate structure, consequently affecting enzyme activity and diversity of microbial functions in soil [23]. Sludge compost utilized for agricultural activities is often used as fertilizers, eventually contributing to MPs content in the soil [24]. Recent studies have shown that fibrous MPs consist of components such as PS, PE and PVC [25]. Also, a major negative impact is observed on plant communities due to the atmospheric deposition flux of MPs [26]. Thus, it can be concluded that the contribution of MPs in our environment can be the result of various pathways, and the regulation of their mitigation can be a great challenge in the coming era.
Status of Indian rivers
India is a developing country and is far from being developed. The inadequate implementation of advanced technologies has resulted in a lack of effective monitoring and regulated control over the release of contaminated industrial effluents into water bodies such as rivers [27]. These water bodies can act as primary conduits for the transportation of MPS from terrestrial to marine ecosystems [28]. The Netravathi River is the largest river in Southern Karnataka, India, which reported 288 particles/kg of MPs [29]. In a study of the holy river of India, i.e., river Ganga, it was found that this magnificent river was filled with MPs concentration (PET and PE) of around 99.27–409.86 particles/kg [30]. Another study alongside the Ganga River stretch of Ballia, Patna, Bhagalpur, Farakka and Diamond Harbour reported various size ranges of MPs [31]. About 134.53–581.70 particles/kg of debris and fiber were found in sediments of the Sabarmati River [32]. In the Brahmaputra River, MPs within the particle size range of 150 μm–5 mm and 20–150 μm exhibited concentrations range from 20 to 240 particles/kg and 531 to 3485 particles/kg, respectively [33]. Similar studies at the Indus River for MPs within a size range of 150 μm–5 mm and 20–150 μm exhibited a concentration range around 60–340 particles/kg and 525–1752 particles/kg, respectively [33]. A study on the Alaknanda River stretch of Uttarakhand region found different types of MP such as high-density polyethylene (HDPE), PVC, low-density polyethylene (LDPE), PP, PET and PS with a size range of 1–5 mm [34]. Figure 4 illustrates the varying microplastic concentrations (microplastic/kg) in different Indian rivers, including Netravathi, Ganga, Sabarmati, Brahmaputra, and Indus. Among them, the Sabarmati River exhibits the highest recorded prevalence of MPs which can be prominently attributed to the proximity of multiple industrial establishments in its vicinity.
Consequences
Aquatic ecosystem
Since plastic production started to begin, the drastic effect of microplastic has been increasing, causing negative impact on aquatic fauna and flora [35]. Studies that have been conducted on this subject have examined aquatic phytoplankton (microalgae), with the majority of them concentrating on the dynamics of phytoplankton growth following exposure to MPs. Some studies have also reported that the exposure to MPs may significantly slow the growth of microalgae [36].
Freshwater microalgae studies revealed that exposure to MPs could alter their genes expression and certain metabolic pathways besides causing a wide range of physical damages and oxidative stressors to the algae cells [37]. The estimated effects of MPs uptake and toxicity are hypothesized to be based on simple process, such as physical adsorption of micro- and even nano-scale plastics. These MPs adhere to the surfaces of algae cells due to which an algal cell may face physical blockage from light and gas exchange [38]. This affect was observed in Chlorella and Scenedesmus where the surface accumulation of positively charged plastic NPs caused a decrease in the activity of their photosynthetic processes [38]. In another study, growth, photosynthetic efficiency and chlorophyll content were all negatively impacted in Skeletonema costatum when it was exposed to PVC microspheres [39]. Investigations using SEM imaging supported the hypothesis that the adverse effects were caused by the microspheres adhering to and accumulating on cell surfaces [39]. Only at a high concentrations (41.5 mg/L), MPs showed a substantial detrimental effect on microalgae (Tetraselmis chuii) growth, while at very low quantities (0.9, 2.1 mg/L), a drop in the chlorophyll count was reported [39].
Marine organisms from different trophic levels have a certain level of MP ingestion [40]. Most of the marine organisms receive MP from seawater or from lower trophic levels [41]. Several aquatic organisms such as bivalves, fishes, zooplanktons, benthic invertebrates, and large marine mammals receive MP as food [42]. MP ingestion depends on the particle size and some extent to physiological and behavioral character of the marine vertebrates and invertebrates [43]. Two shapes of MP, fibers and fragment, are mainly reported in aquatic organisms [44]. Several ecotoxicological effects due to different MPs have been documented in various groups of aquatic organisms as shown in Fig. 5. In aquatic microorganisms, MPs can also enter in circulatory systems [45]. When aquatic organisms ingest MPs, these small particles can easily enter the cells and retain in their tissues. For instance, in the hemolymph of blue mussels, M. edulis, particle size of 9.6 µm has been recorded [46]. Additionally, reduced reproduction capability, body size and neonatal malformation are some other ecotoxicological effects observed in Daphnia magna due to the exposure of nanosized polystyrene (PS) particles [47].
Microplastics have also been found in gills, hemolymph and digestive tissues of Mediterranean mussel (Mytilus galloprovincialis) and have been determined to be responsible for changes in gene expression profile and altered immunological response [48]. Other detrimental effects such as circulatory disorder, inflammation and alteration in intestinal tissues in the species of Girella fish (Girella laevifrons) have also been reported in recent studies [49]. In Nile tilapia (Oreochromis niloticus), it was observed that the mortality of early juvenile tilapia due to anemia and perturbations was caused by MPs [50]. Similarly, mortality of Planktonic crustacean (Daphnia magna) increased due to the alteration of toxicity of pollutants such as herbicides by microplastics [50]. MPs toxicity has also been the cause behind lipid accumulations in liver and inflammations in zebra fishes [51]. This was also evident in a recent study, where an attempt to understand the effects of PE-MPs on Physalaemus cuvieri tadpoles reported that exposure to PE-MPs, along with a mixture of pollutants, certain concentrations of MPs reflected various biochemical and physiological responses [52, 53]. Such a study provides valuable insights into the unexplored effects that MPs exposure can have on amphibians. In fact, swimming capabilities have also been reported to be compromised due to adhesion of MP particles to appendages of copepod [54]. More recently, in Farrer’s scallop (Chlamys farreri) MPs have been determined to cause ultrastructural changes in gills and digestive glands [55]. Thus, MPs have triggered a massive impact on aquatic, both marine and freshwater ecosystems, causing diverse damages in various aquatic life forms. Various recent literature studies have suggested standardizing detection and analysis methods for valid comparisons. Additionally, conducting large-scale experiments is necessary to evaluate ecotoxicological effects of MP and their transport from molecular to ecosystem levels in aquatic biota [56].
Terrestrial ecosystem
Microplastic pollution analysis for terrestrial ecosystem is essential to minimize its negative impact in future. In spite of various concentration limits, which have been imposed by environmental regulations for industrial effluents, MPs continue to flow into the terrestrial ecosystem. In this context, a recent mouse model experiment conducted to evaluate MPs affects has determined a decrease in the number of spermatogenic cells in mice and reported formation of cavities in testicular tissues due to PS-based MPs [57]. This study showed exposure of micro-PS on mice decreased the activity of lactate dehydrogenase (LDH) and succinate dehydrogenase (SDH) enzymes, which are responsible for sperm development and energy production, considerably. Besides this, micro-PS was also reported to aggravate oxidative stress by increasing reactive oxygen species (ROS) and malonaldehyde level in mice [57].
MPs can also accumulate in human beings through various pathways. Sea foods such as fish, marine animal species, and microalgae are primary sources of foods in human diet [58, 59]. Additionally, different brands of salts are used by humans, which provides essential nutrients, as well as acts as a food preservative [60]. In the period 2015–2018, a study showed that MPs were found in 128 brands of commercial salts [61], thus indicating how deep the roots of MPs toxicity may have spread in the food chain. MPs may also enter human body through atmospheric inhalation. Synthetic fabrics, damaged materials (tires, buildings) and the re-suspension of microplastics in surfaces are few sources that cause MPs to be discharged into the air [62]. In the respiratory system, due to large surface area of small particles like MPs, macrophage movement may be disrupted which results in chronic inflammation [63]. Additionally, PE used in packaging of drinking bottle and microwave packaging are, lately, considered as human carcinogens [64]. In their systematic review, Pico et al. assessed the presence, movement and destiny of MPs in raw, processed and bottled water as well as their potential effects on human health [65]. Furthermore, recent studies have reported that MP may be released from long-term usage of contact lenses under sunlight [66], plastic take-out food containers and food storage containers [67, 68], plastic chopping boards [69] and disposable face masks such as those used in COVID-19 [70]. Table 1 presents some of the detrimental impacts of MPs exposure confirmed through different in vivo and in vitro studies. However, more of these harmful effects may have been recorded in the literature over the last few years, and this is only a small selection from the large amount of evidence.
A recent study revealed that MPs contribute to approximately 7% of the total weight of top soil in contaminated areas [81]. This can be attributed to the significant durability of MPs in soil, as a result of limited light and oxygen exposure, allowing them to persist for more than a decade [43]. Porosity or soil may be affected due to MP deposition which ultimately alters the aggregation properties [82]. It has been reported that the vertical migration of MPs is facilitated by large soil particles and dissolved organic materials. The movement potential of polyamide MPs is observed to be the highest, while other MPs such as PE, PET, and PP also show moderate movement potential [83].
But MPs could form channel for water movement in soil, thereby increasing evaporation, it may also desiccate soil surface by destructing soil structure integrity [84]. Eventually, such MPs-contaminated soil poses a significant effect in the case of terrestrial flora, as these MPs have a direct or indirect impact on morphology and physiology of plants [85]. However, generally, uptake of MPs is not favored by plants due to their high molecular weight, and instead, several studies have reported nanosized particles are more easily absorbed by plants and can easily pass through the cell wall [86, 87]. This is illustrated in Fig. 6, which depicts MPs/NPs contaminants in soil can affect plant growth and nutrition in direct and indirect ways. Physiological effects on plants such as hampered growth of wheat in vegetative and reproductive stage have also been reported [88]. Rice plant shows decline in growth, oxidative damages and disrupted gas exchange on exposure to the high dose (3 mg/l) of PS MPs [89]. It was observed that the MPs can adhere to the plant’s root surfaces, resulting in the decrement of water and nutrients uptake [88]. Evidently, few years from now, a significant impact on plant growth and physiology due to long-term exposure of MPs may be seen in terrestrial ecosystems.
Solutions and strategies
Microplastics market is currently a source of global environmental concern, and the recent increase in awareness has generated new avenues for researchers to work on this subject around the world. There is an urgent need for control, remediation and removal of these intoxicating pollutants (MPs) due to the exponentially rising manufacture and use of plastic in our daily lives. Thus, replacing the progressive linear flow model with a circular flow model, such as the reduce, reuse and recycle (3Rs) approach, can lead to a sustainable method of management of MPs in our environment [90].
Wastewater treatment plants
Wastewater treatment plants (WWTPs) often use various steps to remove the toxic particles and micropollutants present in the waste matrix. The concentration of MPs present in different WWTPs can vary with respect to wastewater sources, demographics, lifestyle and economy, sampling and detection methods. Thus, numerous factors can create challenges in the accurate qualitative and quantitative analysis of MPs. Due to this reason, WWTPs require multiple treatment steps, namely primary, secondary and tertiary treatment processes, for obtaining water fit for reuse. The primary step involves the usage of appropriate screens or filters. Nearly 70–98% MPs are believed to be removed through the primary treatment [91]. The secondary treatment, usually, consists of biological process to remove MPs by using appropriate microorganisms. Unfortunately, this treatment displays a moderate efficiency ranging within 0.2–14% [9]. This may be due to the resistance exhibited by MPs to biological decomposition. Finally, tertiary treatment is the last stage which involves removal of other harmful solid matter and inorganic remnants, such as nitrogen and phosphorous. Despite using such a meticulous method, current technology still fails to eliminate all possibilities of MPs leakage. A major cause of this challenge is the continuous mixture of wastewater which homogenously enters the reactors. As a result, the MPs particles that have a density difference do not get sufficient contact time during secondary and tertiary treatment processes. Thus, it is estimated that MPs may persist in treated waters due to the fact that conventional treatment processes were not initially designed with a specific focus on removal of MP pollutants.
It is quite often observed that wastewater plants are situated close to water bodies. These plants discharge large number of MPs into water bodies, thereby requiring a major up-gradation in the technologies incorporated for treatment. It has been reported that sludge from various WWTPs consists of about 4.40 × 103–2.40 × 105 particles of MPs per kg [92]. In addition, the effluents consist of MPs falling within a range of 0.01 particles L−1 to 2.97 × 102 particles L−1, while influent concentration ranged from 0.28 particles L−1 to 3.14 × 104 particles L−1 [92]. This puts a significant emphasis on the WWTPs-generated MPs through sludge or direct discharge into water bodies [90]. So, to further enhance the treatment steps in WWTPs, many techniques have been explored, some of which have been briefly discussed.
Flocculation
The primary phase in treatment of wastewaters is the crucial step for maximum removal of MPs [93]. An essential technique to enhance this phase is through the technique of flocculation or coagulation method (FCM). This method allows the usage of an appropriate floc/coagulant, which on addition to the wastewater can interact with the MPs particles. Lapointe et al. reported that the aggregation of MPs and flocs was observed due to interactive forces such as hydrogen bond, van der Waals forces and electrostatic forces (Fig. 7) [93, 94]. In this method, flocculation followed by settling illustrated that PE, as well as polyesters fibers, could be successfully separated. Iron-based [95] and aluminum-based [96] flocculants/coagulants have been determined to successfully aggregate MPs, while the presence of functional groups [94] (hydroxyl group and carboxyl group) has also enhanced flocculation of MPs. Other advanced hybrid techniques such as electrocoagulation electro-flotation (EC/EF) have also been recently explored as alternatives [97,98,99]. In this method, sacrificial anodes release coagulants and electrolysis occurs at the cathode, where flotation is responsible for the removal of micropollutants.
FCM, however, may be dependent on various factors such as size and shape of MPs, pH, coagulant/floc dosage and chemical properties and other operational parameters [93]. In addition to this, the application of FCM in municipal wastewater matrix must be studied in depth to fully understand and utilize this potentially efficient method.
Ultrafiltration
Membrane-based separation techniques such as ultrafiltration (UF) can also be used to remove MPs. In this method, an appropriate membrane is able to selectively remove desired particles by utilizing the concept of size-based pressure-driven particle capture [100]. It provides two of the major advantages for MPs removal method: low-energy consumption and high MPs removal efficiency. Moreover, this method can be used for compact plant sizes also. It is often used as a replacement for FCM, or in combination with FCM.
In a recent study, two WWTPs (A1 and A2) located in Bangkok, Thailand, were compared such that A2 was equipped with UF as a final step (Fig. 8) [101]. The conventional A1 WWTP exhibited a MP removal efficiency of 78.73%. However, the underground A2 WWTP, which was coupled with pilot-scale UF, showed a MP removal efficiency of 96.97%. A large section of the separated MPs and fibers were determined to fall within a range of 0.05–0.5 mm, which indicates that the high efficiency may be credited to the small size of the particles. Furthermore, the Fourier-Transform Infrared Spectroscopy (FTIR) analysis of most of the separated fibers can indicate the source and composition of removed MPs.
Comparative study of a conventional WWTP and another WWTP coupled with UF in Bangkok, Thailand [101]
Membrane bioreactors
Membrane bioreactor (MBR) is an advanced technology that involves the use of biological catalysts coupled to a separation technique, commonly operated by a membrane [102]. The biological catalysts may be bacteria or enzymes, and common separation techniques, such as UF, may also be incorporated in this method. The presence of a catalysts helps in biodegrading complex organic matter and consequently decreasing the complexity of the waste matrix. The lowered solution complexity permits better MPs removal. As shown in Fig. 9, this process begins as a stream of the waste matrix, which may be pre-treated, flows into the bioreactor. Herein, biodegradation of the organic matter produces a mixed liquid, which further flows into the membrane reactor via a cross-flow filtration system. This leads to the separation of treated water, while the return sludge flows back into the bioreactor for further cycles of treatment.
It provides systematic compartmental units which result in a controlled multi-phase or heterogeneous reaction system. Besides this, other treatment methods can also be integrated with this technique, allowing researchers to uplift the efficiency of WWTPs. Baresel et al. discovered that employing a combination of membrane bioreactor and ultrafiltration with granulated active carbon biofilter can create favorable conditions for detection of MPs present in wastewater effluents [103]. In 2017, Talvitie et al. reported that the secondary treatment of wastewater using MBR can remove MPs as efficiently as 99.9% [104]. As shown in Fig. 10, they reported comparative studies depicting highest efficiency of MBR as compared to other methods such as sand filter, disk filter and flotation method [104].
Although many recent innovations and development have been made for MPs removal from wastewater, the research in this field is still at its preliminary stage. Full-scale application in WWTPs faces major challenges which constitute of unavoidable downsides, such as high cost of inputs, maintenance and filter clogging.
Recycling
Limited science and technology hinder speedy advancements in removal and degradation of MPs, due to which alternatives such as plastic recycling need to be explored simultaneously to boost the formation of a circular economy. Subsequently, a circular economy maximizes economic efficiency, lowers resource consumption and reduces environmental pollution, thereby fostering sustainability and responsible resource management [105]. The four typical strategies of recycling MP waste are primary, secondary, tertiary, and quaternary recycling, which are briefly highlighted below.
-
(I)
Primary recycling: It is an in-plant mechanical recycling method that invests scrap materials directly into prime-grade products without any pre-treatment. This type of recycling is a closed-loop technique which majorly produces high-quality plastics from uncontaminated plastic resources that are often created by manufacturers [106].
-
(II)
Secondary recycling: It is mechanical recycling which involves production of new products from mixed plastic wastes [17]. Unlike primary recycling techniques, secondary recycling is a downgrading technique which generates low-quality plastic from contaminated plastic waste [107]. Collection, sorting, shredding, washing and pelletizing are just a few of the processes involved in mechanical recycling (Fig. 11). It is one of the most commonly used methods of recycling plastics [108].
-
(III)
Tertiary recycling: It is achieved by recovering plastic wastes into value-added products, such as oil or hydrocarbons [17]. This type of recycling is also known as chemical recycling.
-
(IV)
Quaternary Recycling: This method invokes energy recovery by high temperature combustion of plastic wastes; though it is a method that faces major challenges such as emission of greenhouse gases [17].
Following collection, sorting and cleaning of the plastic wastes, there are typically four standard routes for recycling plastic (Fig. 12) [109]. Primary recycling is the use of plastic leftovers to create goods with properties similar to the original material. The term "closed-loop process" is also used to describe this well-established procedure. In secondary recycling, plastic wastes are recovered via mechanical means which include methods such as injection molding and screw extrusion. Plastic waste is transformed into smaller molecules, typically liquids or gases, through tertiary recycling or chemical recycling. These molecules are then used as the feedstock for a process that produces chemicals and fuels. Most commonly pyrolysis (in the presence of catalysts) has been used to mainly obtain three by-products (gases, oils and hydrochar). Quaternary recycling, often known as incineration, involves recovering energy from waste plastic through burning, significantly reducing the volume of waste. This method is typically used when wastes are too contaminated to be recycled normally. However, it produces waste residues which are accompanied by generation of toxic pollutant gases. By adding activated carbon, neutralizing acids, introducing ammonia into the combustion chamber and other methods, hazardous gases that are often released during combustion can be minimized. Waste that is poisonous or contagious can be effectively decomposed since the waste is reduced to roughly 1% of its initial volume. Consequently, this is the best recycling method for medical waste.
Some other common recycling techniques are solvent extraction, gasification, pyrolysis, and hydrothermal processes [110]. The technique of solvent extraction involves dissolving the target polymer in an appropriate polymer-compatible solvent and thereafter extraction is achieved by precipitation. Hydrothermal processes involve the depolymerization of sub or supercritical fluids which show high selectivity. Hydrothermal and solvent extraction has greater application in the case of mixed plastic waste [110]. Gasification provides a platform for generation of fuel from MPs; a technique that has been determined to influence the production efficiency of syngas from a combination of feedstock containing biomass and plastic wastes [111]. Lastly, pyrolysis is carried out in the absence of oxygen, at high temperature (400–800 °C), for the depolymerization of complex polymers to simpler products.
In a recent study, the acid-catalyzed recycle of PS to valuable products was carried out at 1 bar pressure of O2 (Fig. 13) [112]. The disintegration of PS is hypothesized to be caused by the formation of singlet oxygen which acts as the ROS. Consequently, the ROS abstract hydrogen from the tertiary C–H bond, which gives rise to hydroperoxidation. This eventually causes C–C cleavage via radical processes, thus generating new value-added products. This simple process has opened up new perspectives and scope for photolytic and photocatalytic recycling of MaPs and MPs.

Reproduced with permission from reference [112]. Copyright © 2022 American Chemical Society
Chemical recycling of PS via a simple and novel photo-acid-catalyzed method using molecular oxygen.
Despite these wide ranges of available methods, recycling plastic is still hindered by restrictions like high processing costs for relatively inexpensive virgin plastic, contaminated plastic resulting in a limited number of re-cycles and poor recyclability of waste plastic made from textiles, flexible packaging, etc. [107, 113, 114]. Furthermore, not all plastics can be recycled, mixed and tainted. Although degraded polymers can be utilized as feedstock or in energy recovery, they are not suitable for recycling. Additionally, producers require a consistent flow of raw materials of uniform quality, which is occasionally challenging with recycled plastic. Consequently, due to its many advantages and disadvantages, recycling plastic wastes to reduce MPs outflow into our ecosystems is still a contentious topic of discussion. However, recycling plastics is still a primary focus in waste management because it has significant environmental advantages such as preserving natural and energy resources, cutting down emissions from pollutants, minimizing the need for landfills and even boosting local economies.
Degradation
Considering the small size range and our limited ability to detect their presence, the removal of MPs is a challenging hurdle. For any comprehensive, in-depth analysis of MPs, advanced separation and degradation techniques as well as the high cost of labor and manufacture must be taken into consideration. As indicated by Atwood et al., the first and main criterion might be satisfied by understanding the fundamental mechanisms underlying microplastic transport into different ecosystems [115]. This can be followed by identification of appropriate methods for removal from desired ecosystems. However, merely removing MPs is not the ultimate and optimal solution for effectively terminating the life cycle of MP. Instead, it is imperative to explore alternative methodologies that encompass complete degradation of MPs, with the goal of generating value-added products [110]. Through three major routes, namely thermal, photocatalytic degradation, and biodegradation, targeted MPs can be disintegrated to low molecular weight compounds [116].
Thermal degradation
The method of thermal degradation, or pyrolysis, occurs via thermo-oxidative reactions on applying heat to large complex polymers, consequently producing smaller monomers [90]. One such recent work, carried out by Wang et al., displays excellent efficiency of adsorption and thermal degradation of PS-based MPs using Zn-/Mg-modified magnetic biochar (MBC) (Fig. 14) [117]. It was hypothesized that the biochar adsorbents are capable of simultaneously carrying out adsorption and thermal degradation. First, adsorption of MPs is induced by electrostatic interaction between the positively charged Mg and Zn particles, along with the metal-O-MP interaction. Second, the active sites of Zn/Mg MBC engage in the hydrogenation of PS MPs during pyrolysis, resulting in the generation of small liquid products. Simultaneously, the adsorbents are recycled through the thermal treatment [117].
Adsorption followed by thermal treatment for removal of PS-based MPs using Zn-/Mg-modified magnetic biochar (Zn/Mg MBC) [117]
In a more recent work, the application of advanced oxidation processes for degradation of MPs has been explored [118]. The decomposition of ultra-high-molecular-weight PE was accomplished in this study using a hydrothermal coupled Fenton system, which achieves 95.9% weight loss in 16 hours and 75.6% mineralization efficiency in 12 hours (Fig. 15). Their study revealed that this degradation unfolded via a two-step process: (a) opening of chain which is a pivotal step and (b) oxidation which gives rise to carbonyl formation. Additionally, this method was reported to be adept in removing a variety of petroleum-based polymers and maintains high efficiency in real-world aquatic environments. An overview of recent thermal degradation of MPs is summarized in Table 2.

Reproduced with permission from reference [118]. Copyright © 2022, American Chemical Society
Decomposition of ultra-high-molecular-weight polyethylene via hydrothermal coupled Fenton system.
From the literature study, it has been perceived that most often thermal degradation of MPs has been opted for their detection, identification and characterization. Most researchers have tried to explore the possibilities of catalytic pyrolysis for MPs degradation. However, high energy requirement poses as a major drawback. Hence, a more environmentally friendly, economical, and feasible technique of MPs degradation is required, which instigated researchers to explore photocatalysts and biocatalysts.
Photocatalytic degradation
An alternative approach, the photocatalytic degradation, allows breakdown of polymers when irradiated with high-intensity photons such that simpler monomers are derived. The current photocatalysts industries provide a wide range of options for carrying out this method of degradation of MPs. However, only a handful of such photocatalysts have been able to exhibit high efficiency. For instance, a novel hydroxy-rich ultrathin photocatalyst, BiOCl, facilitated degradation of MPs due to enhanced production of surface hydroxyl radicals, as shown in Fig. 16a [120].

Reproduced with permission from reference [120]. Copyright © 2020 Elsevier B.V. All rights reserved. (b) Visible-light-driven degradation of PP generating value-added by-products in a continuous water-flow system. Reproduced with permission from reference [121]. Copyright © 2020 The Author(s). Published by Elsevier B.V. (c) MXene-based photocatalyst used for visible-light-driven H2 production and degradation of PET MPs. Reproduced with permission from reference [122]. Copyright © 2021 Elsevier Inc. All rights reserved
Various photocatalytic degradations: (a) catalyst BiOCl used for photocatalytic degradation of MPs.
An attempt at exploring metal oxide NPs (MONPs) for photocatalytic degradation of MPs have also been carried out. Uheida et al. designed ZnO nanorods adhered onto glass fibers which demonstrated visible-light-driven degradation of PP spherical MPs (Fig. 16b) [121]. Their study reported that upon irradiation for two weeks, the generation of products, such as acetone, butanol, acetaldehyde, and formaldehyde, were additionally observed. These generated by-products exhibit significant potential for utilization in various industrial applications. More recently, due to the multi-fold advantages of photocatalysts, Cao et al. designed MXene/ZnxCd1-xS photocatalysts which successfully exhibited photocatalytic H2 production and PET degradation, as shown in Fig. 16c [122]. Therefore, it is evident that photocatalysts can prove to eliminate MPs from various systems, while simultaneously bringing forth additional benefits such as value-added products and energy generation. An overview of recent photocatalytic techniques is laid out in Table 3.
Biodegradation
An emerging green method of degrading MPs is through the usage of biological catalysts. This process of biodegradation can be carried out by using bacteria, enzymes, fungi, and even larvae. These biocatalysts can simply adsorb MPs as carbon source, as well as degrade MPs to produce simpler non-toxic monomers. For instance, Yoshida et al. reported that novel bacterium Ideonella sakaiensis 201-F6 used PET as a source of carbon and energy to produce two benign monomers [133]. However, biodegradation proves to be a surprising method as polymers pose an inert and recalcitrant nature in our ecosystems. Being a relatively newer approach, with limited knowledge, researchers aim to explore this concept and develop sustainable and reliable methods for bioremediation of MPs using fungi, biofilms, bacteria and bacterial consortiums [134].
Recently, Auta et al. demonstrated that Bacillus cereus and Bacillus gottheilii were able to break down MPs. These bacterial strains consumed MPs by using them as a carbon source [135]. It was observed that Bacillus cereus enzymatic mechanisms cause the weight loss of PE, PET, and PS by 1.6, 6.6 and 7.4%, respectively, while Bacillus gottheilii causes the weight loss of PET, PP, PS, and PE by 3.0, 3.6, 5.8 and 6.2%, respectively. Similarly, Chauhan et al. used Exiguobacterium species for developing biofilms on the surface of PS, which successfully achieved weight loss of 8 and 8.8%, respectively [136]. Yuan et al. reported that fungal strains such as Aspergillus tubingensis, Penicillium simplicissimu, Zalerion maritimum and Aspergillus flavus are also capable of exhibiting efficient MP degradation [134].
Besides microbes, microbial enzymes have also been explored as an alternative, such as lignin peroxidases, proteases and lipases for degradation of PE, PU, and PET, respectively [137,138,139]. However, the intrinsic chemical additives in MPs may reduce the effectiveness of microbial enzymes [140]. Moreover, the usage of enzymes proves to be time-consuming and a costly process. Additionally, in order for microbial enzyme colonies to function well, optimal conditions must be created, which is a difficult process in natural systems. Considering these limitations, microbes are a preferred choice for biodegradation of MPs as they obviate the need for laborious time-consuming procedures involved in the extraction and purification of microbial enzymes. Furthermore, microbes can be efficiently utilized in regenerative cycles, thereby enhancing the overall effectiveness and cost efficiency of biodegradation.
Research has been expanded into insects-mediated biodegradation techniques as well [141]. A recent study shows that Zophobas atratus larvae were successfully able to degrade PS and LDPE without the generation of any NPs (Fig. 17) [142]. Conjointly, enhanced efficiency of biodegradation of MPs may be achieved by pre-treatment through thermal and photoreactive methods. Thus, persistent research and development in the area of MP biodegradation have the potential to significantly accelerate the mitigation of MPs from all ecosystems. An overview of recent biodegradation techniques is laid out in Table 4.

Reproduced with permission from reference [142]. Copyright © 2022 Elsevier Ltd. All rights reserved
Schematics of larval-mediated biodegradation of polystyrene (PS) and low-density polyethylene (LDPE) MPs.
Challenges and future perspectives
With the critical emergence of MPs in the environment, the potential threat of MPs on the ecosystems must be retaliated with upgraded scientific techniques and better waste management strategies. Researchers, government and other public and private bodies are investing their technological and financial assets to foster new rational and strategic designs to effectively eliminate MPs from the environment. The scientific community must strive and yield progresses through advances such as photocatalytic degradation and biodegradation, which can help eradicate MPs from the biosphere. Nevertheless, degradation of MPs is a difficult obstacle due to their narrow size range and our limited capacity to detect them. Properties of MPs such as large surface area and hydrophobicity further allow them to act as potential substrates for other contaminants such as heavy metals and pathogenic microorganisms. Therefore, MPs impose a significant impact on the environment. However, it is impossible to completely ban plastics, and therefore, plastic consumption should be simultaneously coupled with emphasis on changes that must be made to reduce the production and consumption of plastic. Other strategies such as enhancing the correct disposal and recycle of plastic wastes and strengthening the legal framework can also cumulatively aid the elimination of MP mitigation into various ecosystems.
In recent years, several advancements have been made to cease MPs flow into the environment with a probable window for many more such advances. Therefore, some of the critical recommendations to foster new research in this domain are as follows:
-
The effects of MPs on higher organisms are not fully explored and understood. A major section of research may be dedicated in analyzing the toxicity of the chemical additives which leach from plastics.
-
Wastewater treatment remains a major challenge as most techniques have been successful at the pilot scale only. More research can be focused on the full-scale implementation of techniques specifically targeted toward MPs removal in WWTPs.
-
Adoption of novel adsorbents for the adsorption of intermediates products is critical and therefore could modulate product selectivity and play an important role in generation of value-added products.
-
So far, there are several approaches such as filtration, adsorption and biodegradation which are extremely effective. But new approaches are of dire need to completely degrade and convert MPs into non-toxic products. Therefore, emerging processes such as advanced oxidation processes, photocatalysis and bioremediation processes may have a bright future and scope.
Conclusion
Exponential increase in plastic consumption at various levels has been a boon and bane to human life. As compared to the various benefits that plastic brings forth, the affliction of plastic-derived pollution is high. Emerging research on the deteriorating effects of MPs has called upon worldwide attention to eliminate MPs. This work attempts to collate information regarding the source, fate and degradation of MPs. It is evident that the primary and secondary sources of MPs unintentionally flow into the biosphere, gradually contaminating soil, water and the food chain. Subsequently, lower and higher organisms are affected directly or indirectly from MPs contamination. However, their impact on higher levels of life forms needs further investigation. Due to their persistent nature, it is utmost crucial to implement effective MPs degradation and prevention actions and therefore requires focus by the scientific and social community. Wastewater treatment plants are one of the major contributors of MPs. It must adopt advanced and updated methods that specifically target the elimination of MPs. Scientific advancements via degradation methods, such as thermal, photocatalytic and biodegradation, can contribute toward the eradication of MPs in the biosphere. However, the small size range and limited technologies to detect MPs act as the major challenges against the removal and degradation of MPs. Advanced separation and degradation techniques and high cost of labor and manufacture must also be considered as obstacles, which remain due to limited knowledge. In order to effectively degrade and remove MPs from our environment before they pose an unavoidable worldwide hazard to all life forms, reliable, efficient, cost-effective and green technologies must be developed. Major reformations must also be adopted against plastic production and consumptions, via pathways such as education and awareness, plastics disposal management and recycling. Adopting novel and innovative approaches and policies would help develop clean and sustainable society, by diminishing accumulated plastic waste from the environment.
References
PlasticsEurope’s Market Research and Statistics Group (PEMRG) (2019) Plastics—the Facts 2019. https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf
Osman AI, Hosny M, Eltaweil AS et al (2023) Microplastic sources, formation, toxicity and remediation: a review. Environ Chem Lett 21:2129–2169. https://doi.org/10.1007/s10311-023-01593-3
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:25–29. https://doi.org/10.1126/sciadv.1700782
Thompson RC, Olsen Y, Mitchell RP et al (2004) Lost at sea: Where is all the plastic? Science 304:838. https://doi.org/10.1126/science.1094559
Paul MB, Stock V, Cara-Carmona J et al (2020) Micro-and nanoplastics–current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv 2:4350–4367. https://doi.org/10.1039/D0NA00539H
Ramaswamy V, Sharma HR (2011) Plastic bags–Threat to environment and cattle health: a retrospective study from Gondar City of Ethiopia. IIOAB J 2:6–11
Alimba CG, Faggio C (2019) Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol 68:61–74. https://doi.org/10.1016/j.etap.2019.03.001
Hurley R, Woodward J, Rothwell JJ (2018) Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat Geosci 11:251–257. https://doi.org/10.1038/s41561-018-0080-1
Sun J, Dai X, Wang Q et al (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37. https://doi.org/10.1016/j.watres.2018.12.050
Jayasiri HB, Purushothaman CS, Vennila A (2013) Plastic litter accumulation on high-water strandline of urban beaches in Mumbai, India. Environ Monit Assess 185:7709–7719. https://doi.org/10.1007/s10661-013-3129-z
Dai Z, Zhang H, Zhou Q et al (2018) Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environ Pollut 242:1557–1565. https://doi.org/10.1016/j.envpol.2018.07.131
Borrelle SB, Ringma J, Law KL et al (2020) Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369:1515–1518. https://doi.org/10.1126/science.aba3656
Khosrovyan A, Melkonyan H, Rshtuni L et al (2023) Polylactic acid-based microplastic particles induced oxidative damage in brain and gills of goldfish Carassius auratus. Water 15:2133. https://doi.org/10.3390/w15112133
Chen H, Wang Y, Sun X et al (2020) Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 243:125271. https://doi.org/10.1016/j.chemosphere.2019.125271
Ali W, Ali H, Gillani S et al (2023) Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: a review. Environ Chem Lett 21:1761–1786. https://doi.org/10.1007/s10311-023-01564-8
An L, Liu Q, Deng Y et al (2020) Sources of microplastic in the environment. Microplast Terr Environ 95:143–159. https://doi.org/10.1007/698_2020_449
Chen J, Wu J, Sherrell PC et al (2022) How to build a microplastics-free environment: strategies for microplastics degradation and plastics recycling. Adv Sci 9:2103764. https://doi.org/10.1002/advs.202103764
Greenshields J, Schirrmacher P, Hardege JD (2021) Plastic additive oleamide elicits hyperactivity in hermit crabs. Mar Pollut Bull 169:112533. https://doi.org/10.1016/j.marpolbul.2021.112533
Shore EA, Huber KE, Garrett AD, Pespeni MH (2022) Four plastic additives reduce larval growth and survival in the sea urchin Strongylocentrotus purpuratus. Mar Pollut Bull 175:113385. https://doi.org/10.1016/j.marpolbul.2022.113385
Sait STL, Sørensen L, Kubowicz S et al (2021) Microplastic fibres from synthetic textiles: environmental degradation and additive chemical content. Environ Pollut 268:115745. https://doi.org/10.1016/j.envpol.2020.115745
Teuten EL, Saquing JM, Knappe DRU et al (2009) Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B Biol Sci 364:2027–2045. https://doi.org/10.1098/rstb.2008.0284
Yu ZF, Song S, Xu XL et al (2021) Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ Exp Bot 192:104635. https://doi.org/10.1016/j.envexpbot.2021.104635
Hodson ME, Duffus-Hodson CA, Clark A et al (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51:4714–4721. https://doi.org/10.1021/acs.est.7b00635
Bayo J, Olmos S, López-Castellanos J (2021) Assessment of microplastics in a municipal wastewater treatment plant with tertiary treatment: removal efficiencies and loading per day into the environment. Water 13:1339. https://doi.org/10.3390/w13101339
Chen G, Feng Q, Wang J (2020) Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ 703:135504. https://doi.org/10.1016/j.scitotenv.2019.135504
Klein M, Fischer EK (2019) Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci Total Environ 685:96–103. https://doi.org/10.1016/j.scitotenv.2019.05.405
Pandey D, Singh A, Ramanathan A, Kumar M (2021) The combined exposure of microplastics and toxic contaminants in the floodplains of north India: a review. J Environ Manage 279:111557. https://doi.org/10.1016/j.jenvman.2020.111557
He B, Smith M, Egodawatta P et al (2021) Dispersal and transport of microplastics in river sediments. Environ Pollut 279:116884. https://doi.org/10.1016/j.envpol.2021.116884
Amrutha K, Warrier AK (2020) The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci Total Environ 739:140377. https://doi.org/10.1016/j.scitotenv.2020.140377
Sarkar DJ, Das Sarkar S, Das BK et al (2019) Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci Total Environ 694:133712. https://doi.org/10.1016/j.scitotenv.2019.133712
Singh N, Mondal A, Bagri A et al (2021) Characteristics and spatial distribution of microplastics in the lower Ganga River water and sediment. Mar Pollut Bull 163:111960. https://doi.org/10.1016/j.marpolbul.2020.111960
Patel AK, Bhagat C, Taki K, Kumar M (2020) Microplastic vulnerability in the sediments of the Sabarmati River of India. In: Kumar M, Munoz-Arriola F, Furumai H, Chaminda T (eds) Resilience, response, and risk in water systems. Springer, Singapore, pp 127–138
Tsering T, Sillanpää M, Sillanpää M et al (2021) Microplastics pollution in the Brahmaputra River and the Indus River of the Indian Himalaya. Sci Total Environ 789:147968. https://doi.org/10.1016/j.scitotenv.2021.147968
Chauhan JS, Semwal D, Nainwal M et al (2021) Investigation of microplastic pollution in river Alaknanda stretch of Uttarakhand. Environ Dev Sustain 23:16819–16833. https://doi.org/10.1007/s10668-021-01388-y
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Besseling E, Foekema EM, Van Franeker JA et al (2015) Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar Pollut Bull 95:248–252. https://doi.org/10.1016/j.marpolbul.2015.04.007
Lagarde F, Olivier O, Zanella M et al (2016) Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339. https://doi.org/10.1016/j.envpol.2016.05.006
Bhattacharya P, Lin S, Turner JP, Ke PC (2010) Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C 114:16556–16561. https://doi.org/10.1021/jp1054759
Zhang C, Chen X, Wang J, Tan L (2017) Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ Pollut 220:1282–1288. https://doi.org/10.1016/j.envpol.2016.11.005
Tanaka K, Takada H (2016) Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci Rep 6:1–8. https://doi.org/10.1038/srep34351
Barboza LGA, Vethaak AD, Lavorante BRBO et al (2018) Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar Pollut Bull 133:336–348. https://doi.org/10.1016/j.marpolbul.2018.05.047
Su L, Xue Y, Li L et al (2016) Microplastics in taihu lake, China. Environ Pollut 216:711–719. https://doi.org/10.1016/j.envpol.2016.06.036
Horton AA, Walton A, Spurgeon DJ et al (2017) Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141. https://doi.org/10.1016/j.scitotenv.2017.01.190
de Sá LC, Oliveira M, Ribeiro F et al (2018) Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci Total Environ 645:1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207
Shi W, Han Y, Sun S et al (2020) Immunotoxicities of microplastics and sertraline, alone and in combination, to a bivalve species: size-dependent interaction and potential toxication mechanism. J Hazard Mater 396:122603. https://doi.org/10.1016/j.jhazmat.2020.122603
Peng L, Fu D, Qi H et al (2020) Micro-and nano-plastics in marine environment: source, distribution and threats—a review. Sci Total Environ 698:134254. https://doi.org/10.1016/j.scitotenv.2019.134254
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48:12336–12343. https://doi.org/10.1021/es503001d
Avio CG, Gorbi S, Milan M et al (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222. https://doi.org/10.1016/j.envpol.2014.12.021
Ahrendt C, Perez-Venegas DJ, Urbina M et al (2020) Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar Pollut Bull 151:110795. https://doi.org/10.1016/j.marpolbul.2019.110795
Zocchi M, Sommaruga R (2019) Microplastics modify the toxicity of glyphosate on Daphnia magna. Sci Total Environ 697:134194. https://doi.org/10.1016/j.scitotenv.2019.134194
Lu Y, Zhang Y, Deng Y et al (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50:4054–4060. https://doi.org/10.1021/acs.est.6b00183
Bobori DC, Dimitriadi A, Feidantsis K et al (2022) Toxic effects of polyethylene-microplastics on freshwater fish species: implications for human health. Public Heal Toxicol 2:A108. https://doi.org/10.18332/pht/149614
Bobori DC, Dimitriadi A, Feidantsis K et al (2022) Differentiation in the expression of toxic effects of polyethylene-microplastics on two freshwater fish species: size matters. Sci Total Environ 830:154603. https://doi.org/10.1016/j.scitotenv.2022.154603
Della Torre C, Bergami E, Salvati A et al (2014) Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ Sci Technol 48:12302–12311. https://doi.org/10.1021/es502569w
Xia B, Zhang J, Zhao X et al (2020) Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ Pollut 258:113657. https://doi.org/10.1016/j.envpol.2019.113657
Du S, Zhu R, Cai Y et al (2021) Environmental fate and impacts of microplastics in aquatic ecosystems: a review. RSC Adv 11:15762–15784. https://doi.org/10.1039/D1RA00880C
Xie X, Deng T, Duan J et al (2020) Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol Environ Saf 190:110133. https://doi.org/10.1016/j.ecoenv.2019.110133
Li N, Wu X, Zhuang W et al (2020) Fish consumption and multiple health outcomes: umbrella review. Trends Food Sci Technol 99:273–283. https://doi.org/10.1016/j.tifs.2020.02.033
Walkinshaw C, Lindeque PK, Thompson R et al (2020) Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicol Environ Saf 190:110066. https://doi.org/10.1016/j.ecoenv.2019.110066
Pironti C, Ricciardi M, Motta O et al (2021) Microplastics in the environment: Intake through the food web, human exposure and toxicological effects. Toxics 9:1–29. https://doi.org/10.3390/toxics9090224
Peixoto D, Pinheiro C, Amorim J et al (2019) Microplastic pollution in commercial salt for human consumption: a review. Estuar Coast Shelf Sci 219:161–168. https://doi.org/10.1016/j.ecss.2019.02.018
Prata JC, da Costa JP, Lopes I et al (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Donaldson K, Stone V, Gilmour PS et al (2000) Ultrafine particles: mechanisms of lung injury. Philos Trans R Soc A Math Phys Eng Sci 358:2741–2749. https://doi.org/10.1098/rsta.2000.0681
Wc LI, Tse HF, Fok L (2016) Plastic waste in the marine environment: a review of sources, occurrence and effects. Sci Total Environ 566:333–349. https://doi.org/10.1016/j.scitotenv.2016.05.084
Picó Y, Manzoor I, Soursou V, Barceló D (2022) Microplastics in water, from treatment process to drinking water: analytical methods and potential health effects. Water Emerg Contam Nanoplastics 1:13. https://doi.org/10.20517/wecn.2022.04
Liu Y, Ling X, Jiang R et al (2023) High-content screening discovers microplastics released by contact lenses under sunlight. Environ Sci Technol 57:8506–8513. https://doi.org/10.1021/acs.est.3c01601
Hu J-L, Duan Y, Zhong H-N et al (2023) Analysis of microplastics released from plastic take-out food containers based on thermal properties and morphology study. Food Addit Contam Part A 40:305–318. https://doi.org/10.1080/19440049.2022.2157894
Aslam S, Khurram A, Hussain R et al (2023) Sources, distribution, and incipient threats of polymeric microplastic released from food storage plastic materials. Environ Monit Assess 195:638. https://doi.org/10.1007/s10661-023-11242-5
Yadav H, Khan MRH, Quadir M et al (2023) Cutting boards: An overlooked source of microplastics in human food? Environ Sci Technol 57:8225–8235. https://doi.org/10.1021/acs.est.3c00924
Cabrejos-Cardeña U, De-la-Torre GE, Dobaradaran S, Rangabhashiyam S (2023) An ecotoxicological perspective of microplastics released by face masks. J Hazard Mater 443:130273. https://doi.org/10.1016/j.jhazmat.2022.130273
Gautam R, Jo J, Acharya M et al (2022) Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines. Sci Total Environ 838:156089. https://doi.org/10.1016/j.scitotenv.2022.156089
Goodman KE, Hua T, Sang Q-XA (2022) Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega 7:34136–34153. https://doi.org/10.1021/acsomega.2c03453
Zhang Y, Wang S, Olga V et al (2022) The potential effects of microplastic pollution on human digestive tract cells. Chemosphere 291:132714. https://doi.org/10.1016/j.chemosphere.2021.132714
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep 7:1–10. https://doi.org/10.1038/srep46687
Stock V, Böhmert L, Lisicki E et al (2019) Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol 93:1817–1833. https://doi.org/10.1007/s00204-019-02478-7
Luo T, Zhang Y, Wang C et al (2019) Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut 255:113122. https://doi.org/10.1016/j.envpol.2019.113122
Chen S, Yang J-L, Zhang Y-S et al (2023) Microplastics affect arsenic bioavailability by altering gut microbiota and metabolites in a mouse model. Environ Pollut 324:121376. https://doi.org/10.1016/j.envpol.2023.121376
Xie L, Chen T, Liu J et al (2022) Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice. Ecotoxicol Environ Saf 246:114194. https://doi.org/10.1016/j.ecoenv.2022.114194
Zaheer J, Kim H, Ko IO et al (2022) Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ Int 161:107121. https://doi.org/10.1016/j.envint.2022.107121
Abdel-Zaher S, Mohamed MS, Sayed AE-DH (2023) Hemotoxic effects of polyethylene microplastics on mice. Front Physiol 14:1072797. https://doi.org/10.3389/fphys.2023.1072797
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50:5774–5780. https://doi.org/10.1021/acs.est.6b00816
Wang W, Ge J, Yu X, Li H (2020) Environmental fate and impacts of microplastics in soil ecosystems: progress and perspective. Sci Total Environ 708:134841. https://doi.org/10.1016/j.scitotenv.2019.134841
Gao J, Pan S, Li P et al (2021) Vertical migration of microplastics in porous media: multiple controlling factors under wet-dry cycling. J Hazard Mater 419:126413. https://doi.org/10.1016/j.jhazmat.2021.126413
Wan Y, Wu C, Xue Q, Hui X (2019) Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci Total Environ 654:576–582. https://doi.org/10.1016/j.scitotenv.2018.11.123
Kumari A, Rajput VD, Mandzhieva SS et al (2022) Microplastic pollution: an emerging threat to terrestrial plants and insights into its remediation strategies. Plants 11:340. https://doi.org/10.3390/plants11030340
Wang W, Yuan W, Xu EG et al (2022) Uptake, translocation, and biological impacts of micro(nano)plastics in terrestrial plants: progress and prospects. Environ Res 203:111867. https://doi.org/10.1016/j.envres.2021.111867
Li H, Zhang L, Lu H et al (2020) Macro-/nanoporous Al-doped ZnO/cellulose composites based on tunable cellulose fiber sizes for enhancing photocatalytic properties. Carbohydr Polym 250:116873. https://doi.org/10.1016/j.carbpol.2020.116873
Liu Y, Guo R, Zhang S et al (2022) Uptake and translocation of nano/microplastics by rice seedlings: evidence from a hydroponic experiment. J Hazard Mater 421:126700. https://doi.org/10.1016/j.jhazmat.2021.126700
Ma J, Aqeel M, Khalid N et al (2022) Effects of microplastics on growth and metabolism of rice (Oryza sativa L.). Chemosphere 307:135749. https://doi.org/10.1016/j.chemosphere.2022.135749
Vaid M, Sarma K, Gupta A (2021) Microplastic pollution in aquatic environments with special emphasis on riverine systems: current understanding and way forward. J Environ Manage 293:112860. https://doi.org/10.1016/j.jenvman.2021.112860
Talvitie J, Mikola A, Setälä O et al (2017) How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res 109:164–172. https://doi.org/10.1016/j.watres.2016.11.046
Liu W, Zhang J, Liu H et al (2021) A review of the removal of microplastics in global wastewater treatment plants: characteristics and mechanisms. Environ Int 146:106277. https://doi.org/10.1016/j.envint.2020.106277
Rajala K, Grönfors O, Hesampour M, Mikola A (2020) Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res 183:116045. https://doi.org/10.1016/j.watres.2020.116045
Lapointe M, Farner JM, Hernandez LM, Tufenkji N (2020) Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environ Sci Technol 54:8719–8727. https://doi.org/10.1021/acs.est.0c00712
Ma B, Xue W, Ding Y et al (2019) Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J Environ Sci 78:267–275. https://doi.org/10.1016/j.jes.2018.10.006
Ma B, Xue W, Hu C et al (2019) Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J 359:159–167. https://doi.org/10.1016/j.cej.2018.11.155
Rahman NA, Jol CJ, Linus AA, Ismail V (2021) Emerging application of electrocoagulation for tropical peat water treatment: a review. Chem Eng Process Intensif 165:108449. https://doi.org/10.1016/j.cep.2021.108449
Dizge N, Akarsu C, Ozay Y et al (2018) Sono-assisted electrocoagulation and cross-flow membrane processes for brewery wastewater treatment. J Water Process Eng 21:52–60. https://doi.org/10.1016/j.jwpe.2017.11.016
Deveci EÜ, Akarsu C, Gönen Ç, Özay Y (2019) Enhancing treatability of tannery wastewater by integrated process of electrocoagulation and fungal via using RSM in an economic perspective. Process Biochem 84:124–133. https://doi.org/10.1016/j.procbio.2019.06.016
Hussain CM, Paulraj MS, Nuzhat S (2022) Chapter 2-Source reduction, waste minimization, and cleaner technologies. In: Hussain CM, Paulraj MS, Nuzhat SBT-SR and WM (eds). Elsevier, pp 23–59
Tadsuwan K, Babel S (2022) Unraveling microplastics removal in wastewater treatment plant: a comparative study of two wastewater treatment plants in Thailand. Chemosphere 307:135733. https://doi.org/10.1016/j.chemosphere.2022.135733
Poerio T, Piacentini E, Mazzei R (2019) Membrane processes for microplastic removal. Molecules 24:4148. https://doi.org/10.3390/molecules24224148
Baresel C, Harding M, Fang J (2019) Ultrafiltration/granulated active carbon-biofilter: efficient removal of a broad range of micropollutants. Appl Sci 9:710. https://doi.org/10.3390/app9040710
Talvitie J, Mikola A, Koistinen A, Setälä O (2017) Solutions to microplastic pollution–removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123:401–407. https://doi.org/10.1016/j.watres.2017.07.005
Yang M, Chen L, Wang J et al (2023) Circular economy strategies for combating climate change and other environmental issues. Environ Chem Lett 21:55–80. https://doi.org/10.1007/s10311-022-01499-6
Prata JC, Silva ALP, Da Costa JP et al (2019) Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution. Int J Environ Res Public Health 16:2411. https://doi.org/10.3390/ijerph16132411
Walker TR, Xanthos D (2018) A call for Canada to move toward zero plastic waste by reducing and recycling single-use plastics. Resour Conserv Recycl 133:99–100. https://doi.org/10.1016/j.resconrec.2018.02.014
Nizamuddin S, Boom YJ, Giustozzi F (2021) Sustainable polymers from recycled waste plastics and their virgin counterparts as Bitumen modifiers: a comprehensive review. Polymers (Basel) 13:3242. https://doi.org/10.3390/polym13193242
Joseph B, James J, Kalarikkal N, Thomas S (2021) Recycling of medical plastics. Adv Ind Eng Polym Res 4:199–208. https://doi.org/10.1016/j.aiepr.2021.06.003
Reimonn G, Lu T, Gandhi N, Chen W-T (2019) Review of microplastic pollution in the environment and emerging recycling solutions. J Renew Mater 7:1251–1268. https://doi.org/10.32604/jrm.2019.08055
Ramos A, Rouboa A (2017) Rescuing the environment: turning (micro) plastics into energy through gasification. U Porto J Eng 3:10–23. https://doi.org/10.24840/2183-6493_003.002_0002
Huang Z, Shanmugam M, Liu Z et al (2022) Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J Am Chem Soc 144:6532–6542. https://doi.org/10.1021/jacs.2c01410
Al-Salem SM, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag 29:2625–2643. https://doi.org/10.1016/j.wasman.2009.06.004
Liu Z, Adams M, Walker TR (2018) Are exports of recyclables from developed to developing countries waste pollution transfer or part of the global circular economy? Resour Conserv Recycl 136:22–23. https://doi.org/10.1016/j.resconrec.2018.04.005
Atwood EC, Falcieri FM, Piehl S et al (2019) Coastal accumulation of microplastic particles emitted from the Po River, Northern Italy: comparing remote sensing and hydrodynamic modelling with in situ sample collections. Mar Pollut Bull 138:561–574. https://doi.org/10.1016/j.marpolbul.2018.11.045
Ghosh SK, Pal S, Ray S (2013) Study of microbes having potentiality for biodegradation of plastics. Environ Sci Pollut Res 20:4339–4355. https://doi.org/10.1007/s11356-013-1706-x
Wang J, Sun C, Huang Q-X et al (2021) Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J Hazard Mater 419:126486. https://doi.org/10.1016/j.jhazmat.2021.126486
Hu K, Zhou P, Yang Y et al (2022) Degradation of microplastics by a thermal fenton reaction. ACS ES&T Eng 2:110–120. https://doi.org/10.1021/acsestengg.1c00323
Liu X, Tian K, Chen Z et al (2023) Online TG-FTIR-MS analysis of the catalytic pyrolysis of polyethylene and polyvinyl chloride microplastics. J Hazard Mater 441:129881. https://doi.org/10.1016/j.jhazmat.2022.129881
Jiang R, Lu G, Yan Z et al (2021) Microplastic degradation by hydroxy-rich bismuth oxychloride. J Hazard Mater 405:124247. https://doi.org/10.1016/j.jhazmat.2020.124247
Uheida A, Mejía HG, Abdel-Rehim M et al (2021) Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J Hazard Mater 406:124299. https://doi.org/10.1016/j.jhazmat.2020.124299
Cao B, Wan S, Wang Y et al (2022) Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J Colloid Interface Sci 605:311–319. https://doi.org/10.1016/j.jcis.2021.07.113
Maulana DA, Ibadurrohman M, Slamet, (2021) Synthesis of nano-composite Ag/TiO2 for polyethylene microplastic degradation applications. IOP Conf Ser Mater Sci Eng 1011:012054. https://doi.org/10.1088/1757-899X/1011/1/012054
Tofa TS, Ye F, Kunjali KL, Dutta J (2019) Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalysts 9:819. https://doi.org/10.3390/catal9100819
Kamalian P, Khorasani SN, Abdolmaleki A et al (2020) Toward the development of polyethylene photocatalytic degradation. J Polym Eng 40:181–191. https://doi.org/10.1515/polyeng-2019-0230
Nabi I, Bacha A-U-R, Li K et al (2020) Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 23:101326. https://doi.org/10.1016/j.isci.2020.101326
Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V et al (2019) New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram Int 45:9618–9624. https://doi.org/10.1016/j.ceramint.2018.10.208
Ariza-Tarazona MC, Villarreal-Chiu JF, Hernández-López JM et al (2020) Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: effect of pH and temperature in the photocatalytic degradation process. J Hazard Mater 395:122632. https://doi.org/10.1016/j.jhazmat.2020.122632
Yuwendi Y, Ibadurrohman M, Setiadi S, Slamet S (2022) Photocatalytic degradation of polyethylene microplastics and disinfection of E. coli in water over Fe-and Ag-modified TiO2 nanotubes. Bull Chem React Eng Catal 17:263–277. https://doi.org/10.9767/bcrec.17.2.13400.263-277
Acuña-Bedoya JD, Luévano-Hipólito E, Cedillo-González EI et al (2021) Boosting visible-light photocatalytic degradation of polystyrene nanoplastics with immobilized CuxO obtained by anodization. J Environ Chem Eng 9:106208. https://doi.org/10.1016/j.jece.2021.106208
Zhou D, Luo H, Zhang F et al (2022) Efficient photocatalytic degradation of the persistent PET fiber-based microplastics over Pt nanoparticles decorated N-doped TiO2 nanoflowers. Adv Fiber Mater 4:1094–1107. https://doi.org/10.1007/s42765-022-00149-4
Zhou D, Wang L, Zhang F et al (2022) Feasible degradation of polyethylene terephthalate fiber-based microplastics in alkaline media with Bi2O3@N-TiO2 Z-scheme photocatalytic system. Adv Sustain Syst 6:2100516. https://doi.org/10.1002/adsu.202100516
Yoshida S, Hiraga K, Takehana T et al (2016) Response to comment on “A bacterium that degrades and assimilates poly (ethylene terephthalate).” Science 353:759. https://doi.org/10.1126/science.aaf8625
Yuan J, Ma J, Sun Y et al (2020) Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ 715:136968. https://doi.org/10.1016/j.scitotenv.2020.136968
Auta HS, Emenike CU, Fauziah SH (2017) Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559. https://doi.org/10.1016/j.envpol.2017.09.043
Chauhan D, Agrawal G, Deshmukh S et al (2018) Biofilm formation by Exiguobacterium sp. DR11 and DR14 alter polystyrene surface properties and initiate biodegradation. RSC Adv 8:37590–37599. https://doi.org/10.1039/C8RA06448B
Krueger MC, Harms H, Schlosser D (2015) Prospects for microbiological solutions to environmental pollution with plastics. Appl Microbiol Biotechnol 99:8857–8874. https://doi.org/10.1007/s00253-015-6879-4
Loredo-Treviño A, Gutiérrez-Sánchez G, Rodríguez-Herrera R, Aguilar CN (2012) Microbial enzymes involved in polyurethane biodegradation: a review. J Polym Environ 20:258–265. https://doi.org/10.1007/s10924-011-0390-5
Wei R, Zimmermann W (2017) Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb Biotechnol 10:1308–1322. https://doi.org/10.1111/1751-7915.12710
Wong JKH, Lee KK, Tang KHD, Yap P-S (2020) Microplastics in the freshwater and terrestrial environments: prevalence, fates, impacts and sustainable solutions. Sci Total Environ 719:137512. https://doi.org/10.1016/j.scitotenv.2020.137512
Peng B-Y, Sun Y, Zhang X et al (2023) Unveiling the residual plastics and produced toxicity during biodegradation of polyethylene (PE), polystyrene (PS), and polyvinyl chloride (PVC) microplastics by mealworms (Larvae of Tenebrio molitor). J Hazard Mater 452:131326. https://doi.org/10.1016/j.jhazmat.2023.131326
Peng B-Y, Sun Y, Wu Z et al (2022) Biodegradation of polystyrene and low-density polyethylene by Zophobas atratus larvae: fragmentation into microplastics, gut microbiota shift, and microbial functional enzymes. J Clean Prod 367:132987. https://doi.org/10.1016/j.jclepro.2022.132987
Sun Y, Shaheen SM, Ali EF et al (2022) Enhancing microplastics biodegradation during composting using livestock manure biochar. Environ Pollut 306:119339. https://doi.org/10.1016/j.envpol.2022.119339
Alaa S (2019) Biodegradation of Polyethylene LDPE plastic waste using Locally Isolated Streptomyces sp. J Pharm Sci Res 11:1333–1339. https://doi.org/10.1088/1755-1315/1057/1/012016
Chen Z, Zhao W, Xing R et al (2020) Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J Hazard Mater 384:121271. https://doi.org/10.1016/j.jhazmat.2019.121271
Zhang J, Gao D, Li Q et al (2020) Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci Total Environ 704:135931. https://doi.org/10.1016/j.scitotenv.2019.135931
Nanthini Devi K, Raju P, Santhanam P et al (2021) Biodegradation of low-density polyethylene and polypropylene by microbes isolated from Vaigai River, Madurai, India. Arch Microbiol 203:6253–6265. https://doi.org/10.1007/s00203-021-02592-0
Kumar S, Teotia UVS, Singh Y (2017) Screening of poly vinyl chloride degrading bacteria from plastic contaminated area of Baddi. J Appl Pharm Res 5:34–37
Pinchi JE, Galvez JO, Castaneda-Olivera CA, Alfaro EGB (2022) Environmental biotechnology: biodegradation of microplastics with larvae of Tenebrio Molitor and Galleria mellonella. Chem Eng Trans 93:187–192. https://doi.org/10.3303/CET2293032
Maleki Rad M, Moghimi H, Azin E (2022) Biodegradation of thermo-oxidative pretreated low-density polyethylene (LDPE) and polyvinyl chloride (PVC) microplastics by Achromobacter denitrificansEbl13. Mar Pollut Bull 181:113830. https://doi.org/10.1016/j.marpolbul.2022.113830
Acknowledgements
One of the authors, Shikha Jyoti Borah, would like to thank Jawaharlal Nehru University, New Delhi, for providing financial assistance. Author Sanjeev Kumar thanks Council of Scientific and Industrial Research (file no. 08/694(0004)/2018-EMR-I) for Senior Research Fellowship.
Funding
Open access funding provided by Durban University of Technology.
Author information
Authors and Affiliations
Contributions
The following authors are involved in the various contributions: Shikha Jyoti Borah (SJB), Abhijeet Kumar Gupta (AKG), Akanksha Gupta (AG), Bhawna (B), Sanjeev Kumar (SK4), Ritika Sharma (RS), Ravinder Kumar (RK), Pramod Kumar (PK), Kashyap Kumar Dubey (KKD), Sandeep Kaushik (SK9), Ajay Kumar Mishra (AKM) and Vinod Kumar (VK). AG, VK and PK were involved in conceptualization; AG, VK, SJB, AKG and RK were involved in methodology; PK, B, KKD, SK4, SK9 and AKM were involved in validation; SJB, AKG and RS were involved in software; SJB, AKG, B, SK4 and RS were involved in investigation; SJB and AKG were involved in resources; SJB, AKG, B, SK4, RS and VK were involved in writing—original draft; AG, SK9, RK, AKM and KKD were involved in writing—review and editing; PK and VK were involved in supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borah, S.J., Gupta, A.K., Gupta, A. et al. Grasping the supremacy of microplastic in the environment to understand its implications and eradication: a review. J Mater Sci 58, 12899–12928 (2023). https://doi.org/10.1007/s10853-023-08806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08806-8