Abstract
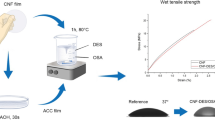
The development of a cellulose nanofibrils film with permanent hydrophobicity using green processes, avoiding hazardous solvents, through easy procedures, is a great challenge. The hydrophobicity of a layer of calcium carbonate modified with stearic acid has already been presented. However, the combination of a cellulose nanofibrils film with a layer of modified calcium carbonate to develop a permanent hydrophobic cellulose-based material rises the additional issue of adhesion between layers. In the present study, a set of cellulose nanofibrils films was coated with a layer of stearic acid and another set was additionally covered with modified precipitated calcium carbonate (0.4–6 µm sized particles with above 50% aragonite crystalline form), previously modified with a stearic acid suspension using ultrasounds. To investigate the issue of adhesion between layers, some films were subjected to heat treatments at 68 and 105 °C. Structural and physical analysis of the films, as well as barrier properties and static/dynamic contact angle measurements were performed. Results show that overall mechanical performance of the films was not substantially affected by the coating and posterior heat treatments. Heat treatments decreased the water vapor transmission rate of stearic acid coated films from 91.9 to 31.6 g m−2 day−1 and the oxygen permeability of stearic acid and modified calcium carbonate coated films from 26.4 to 2.6 cm3 µm/(m2 day kPa). The double layered coated cellulose nanofibrils films attained contact angle hysteresis of 3.1° and 5° and static contact angles of 150° and 140° with no heat treatment and with a heat treatment of 68 °C, respectively. The heat treatment enabled to permanently adhere modified calcium carbonate particles on the film, providing it with persistent hydrophobicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In line with the principles of Green Chemistry we should strive to use renewable feedstock and raw materials, design safe chemicals and products and use safe solvents and reactions conditions [1,2,3]. Out of the renewable materials, cellulose is maybe the most attractive due to its abundance, biodegradability, and non-toxicity [4]. Cellulose nanofibrils (CNFs) have recently been the subject of much interest due to their unique characteristics such as very high aspect ratio, high specific strength, thermal stability, hydrophilicity and broad capacity for chemical modification, among others [2, 5, 6]. These attributes make this material suitable for many industrial applications [7]: for instance, as nanofibrils have the ability to form very dense and entangled networks, freestanding CNF films are a promising platform for the development of food packaging materials [6, 8]. However, the hydrophilicity of cellulose makes it sensitive to moisture, thus often limiting its use where high relative humidity is required. Consequently, extensive research has been devoted to increasing the hydrophobicity of cellulosic materials and enhancing their barrier properties in wet or humid conditions [4, 9,10,11,12,13,14].
Several methods have reported increasing the hydrophobicity of CNF films, including chemical modification of the cellulose fibrils [13, 15]. Despite the efforts on applying green systems, hydrophobization of cellulose through chemical modification routes usually requires the addition of organic solvents and some environmentally unfriendly reagents [16].
Other methods involve either the incorporation of a hydrophobic component in the structure [17] or coating cellulose-based structures with hydrophobic components [4, 6].
Besides reducing the surface energy, many authors have emphasized a multi-scale roughness profile as an important contributor to the hydrophobic properties of a materials’ surface, since it increases the total surface area and allows for the entrapment of air in between the water droplets and the surface. Depending on the wetting regime, the water droplets can either penetrate in between the roughness peaks or remain on top of them [18].
Mertaniemi et al. have successfully super-hydrophobized surfaces with spray-dried chemically modified CNFs leading to hierarchical surface roughness, closely resembling that of lotus leaves [19]. Huang et al. have reported a sprayable super-hydrophobic coating produced from a CNF ethanol suspension [20]. Arbatan et al. reported the preparation of a superhydrophobic paper through dip coating a filter paper in an aqueous suspension containing precipitated calcium carbonate (PCC) and CNFs, to increase the papers’ surface roughness and a subsequent chemical treatment with a solution of alkyl ketene dimer in n-heptane [21].
While in these last reports, much effort has been paid to attaining nanosized roughness via coating with CNFs, other authors on the other hand, have focused on the hydrophobization through hydrophobic modification of coatings and their adhesion on the surfaces. For instance, Hu et al. have reported a combination of surface coating with a dipping treatment in order to increase the water contact angle to near 150° and water resistance of regular paper, using PCC for roughness control, stearic acid (SA) as a surface hydrophobic modification agent and latex as a polymer binder [22].
Unlike some authors, who have synthesized hydrophobic PCC particles [23,24,25], in the present work the PCC’s surface was modified with stearic acid through a simple and rapid method (the used PCC was mostly under aragonite crystalline form, which is more hydrophilic than calcite). Though such hydrophobic modification of PCC and coating techniques have been applied to paper, as far as we are aware of, they have not been applied on CNF films aiming the increase in hydrophobicity. Besides, while current procedures for manufacture of micro- and nano-scale roughness in hydrophobic surfaces are difficult at an industrial large-scale and the chemicals used to provide hydrophobicity are often expensive and toxic, this method comes as a simple and relatively inexpensive process for increasing the hydrophobicity of CNF films without using hazardous solvents or time-consuming methods, as well disregarding the need of a binding agent between the CNF films and the hydrophobic coating.
In the present work, CNF freestanding films were manufactured though vacuum filtration and were hydrophobized with a layer of stearic acid. Also, a layer-by-layer technique with stearic acid and a modified PCC hydrophobic powder, combined with heat treatment was performed.
Materials and methods
A commercial Totally Chlorine Free (TCF) never dried bleached kraft eucalypt pulp (with an average limiting viscosity of 570 mL/g) was used as a source of native cellulose fibers. Stearic acid (SA), 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO), sodium hypochlorite, sodium bromide and other reported chemicals were of laboratory grade (Sigma-Aldrich, USA) and were used without further purification. Precipitated calcium carbonate (PCC) (Sturcal® F) with a minimum of 50% aragonite crystalline form content, an apparent density of 0.45–0.56 g/cm3 and particle size in the range 0.4–6 µm (values as reported by supplier) was provided by J.M. Pereira (Portugal).
CNF suspensions production
A commercial TCF never dried bleached kraft eucalypt pulp (around 80% cellulose and 20% xylan) was used to produce a CNF aqueous suspension. The pulp was subjected to a 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO)-mediated oxidation pretreatment based on previously reported reaction conditions [26], as follows: 10 g oved dried of pulp fibers were resuspended in a total volume of 3 L of 50 mM sodium carbonate buffer solution with a pH of 10.5, containing 0.025 g of TEMPO and 0.25 g of NaBr. A total of 80 mmol of NaClO were added to the previous mixture divided into 4 equal parts, with 15 min apart between each addition. The mixture was then allowed to react for an additional 60 min, all while in continuous agitation and at room temperature. The reaction was then stopped by vacuum filtrating the resulting fibers in a porous plate funnel and washing them with distilled water until pH 7 was achieved.
The resulting fibers were afterward resuspended in distilled water at a solid content of 1.5 wt% and were subjected to 2 successive homogenization steps (500 bar + 850 bar), using a GEA Niro Soavi (Panther NS3006L; GEA, Parma, Italy).
Microscopy observations
In order to morphologically characterize the cellulose fibrils in the obtained CNF suspension, a drop of 0.01 wt% CNF suspension was allowed to air dry overnight at room temperature on a scanning electron microscope (SEM) sample holder. The CNF films surface imaging was performed by attaching fragments directly on the SEM sample holder with double sided tape. Transversal cuts of the coated and non-coated CNF films were also made into representative samples, and they were attached vertically on the sample holders so as to observe their cross section.
Microscopic observations were performed using either a field emission scanning electron microscope (FE-SEM) (Hitachi SEM SU-70 Hitachi, Tokyo, Japan) operated at 15 kV or SEM (Hitachi S-2700; Hitachi, Tokyo, Japan) operated at 20 kV. All of the samples were previously gold sputtered by cathodic spraying (Quorum Q150R ES; Quorum Technologies, East Sussex, UK).
For the transmission electron microscope (TEM) imaging, drops of 0.001 wt% CNF suspension were deposited on carbon-coated electron microscopic grids and negatively stained with 2 wt% uranyl acetate. The grids were air-dried and analyzed with a Hitachi HT-7700 TEM (Hitachi, Tokyo, Japan) with an acceleration voltage of 80 kV.
Degree of polymerization
The CNFs limiting viscosity [η] was determined according to the ISO 5351 (2012) standard, with a cupriethylenediamine (CED) solution as a solvent and using a capillary viscometer. The degree of polymerization (DP) was calculated using the following Eqs. (1, 2) [27].
where is [η] is the limiting viscosity (mL/g) and DP is the degree of polymerization.
Total acidic groups content
The total acidic groups content in the produced CNFs was determined through a conductivity titration method, based on the standard SCAN-CM 65:02. Initially, the CNFs were protonated by resuspension in a HCl solution and then they were washed by sequential cycles of centrifugations, at 3000 g for 15 min, and resuspension in fresh distilled water, until the supernatant attained a conductivity of 5 μS/cm maximum. The CNF suspensions were afterward titrated with a NaOH solution until pH = 11. The amount of weak acid groups was determined from the break points in the conductivity versus added volume of NaOH from the curves obtained from the conductivity titrations.
Preparation of stearic acid suspension and modified PCC
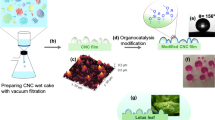
In a first instance, a stearic acid (SA) aqueous dispersion was prepared using a method adapted from Lozhechnikova et al.: SA was added to distilled water at a concentration of 0.4 wt% and the mixture was heated up on a hot plate until it reached 90 °C. At that point, the hot mixture was sonicated at 20 kHz for 3 min with 2 s interval using an ultrasonic liquid processor equipped with a CV17 probe (Vibra-Cell VC 600-2, Sonics & Materials Danbury, USA) and was afterward transferred to an ice bath and let to cool down. The final dispersion was filtered through a filtering funnel with 40–100 μm nominal maximal pore size [28]. The filtrate was a stable milky looking SA dispersion.
In a second stage, a modified PCC powder was prepared using the following method, described by Arbatan et al.: A 0.7 wt% PCC aqueous suspension was prepared submitting it to homogenization in a mild ultrasound bath (S30H, Elmasonic, Singen, Germany) for 3 min immediately before use [21]. Then, 4 mL of this suspension (28 mg of PCC) was added to 10 mL of the previously prepared SA dispersion (≈ 40 mg of SA), and the mixture was subjected to sonication in the ultrasonic liquid processor for 3 min, followed by overnight water evaporation in an oven at 105 °C. This resulted in a dry white modified PCC powder. The modified PCC powder was manually ground in mortar and pestle to disintegrate lumps and obtain a fine powder which was used afterward as a film coating without further treatment.
Production and coating of CNF films
The previously produced CNF suspension was diluted to a solid content of 0.3 wt% and homogenized (Ultra-Turrax T-25 Digital Homogenizer, Ika Labortechnik, Staufen, Germany) for 2 min at 6000 rpm. The resulting suspension was subjected to a mild ultrasound bath (S30H, Elmasonic, Singen, Germany) for 5 min to release the air bubbles.
Simple CNF films with a diameter of 8.5 cm and a target grammage of 55 g/m2 were prepared by vacuum filtration in a filtrating funnel, using a 0.65 μm pore size nitrocellulose membrane (Millipore, Bedford, Mass., USA) as filtration medium, attached to a filter paper for added support. Vacuum filtration was carried out at a constant pressure of 0.2 bar, using a vacuum pump, until the water stopped draining, which took around 180 min. Then, the wet CNF filtration cake and filtrating membrane were placed between two pieces of cloth and blotting paper (one cloth piece on each side), and the whole set was pressed for 30 s at 2.19 × 105 N/m2 in order to remove some of the remaining water and ease the membrane detachment. Then, after carefully removing the membrane, and while still holding the piece of cloth on the opposite side in direct contact with the CNFs, a metallic disk was adhered, and the set was once again pressed for an additional 10 s at the same pressure, just enough for the CNFs to adhere to the metallic disk. Finally, the cloth was gently removed from the top and the CNF film was dried overnight, between perforated metallic rings under pressure applied to the edge of the films in order to prevent them from shrinking, in a standard atmosphere for conditioning (23 °C and 50% of relative humidity). These uncoated CNF films will be designated as CNF from now on.
A second set of SA layered CNF films were produced as follows: the CNF suspension was vacuum filtered under the same previously described conditions. At the end of the water drainage, 3.9 mL of the SA suspension, corresponding to 5 wt% of the film’s total dry mass were diluted to a total volume of 10 mL to ensure coverage of the entire filtration cake’s surface. The suspension was added on top of the CNF filtration cake, and it was once again let to vacuum filter until the water stopped draining, which took an additional 15 min. The following steps of pressing and drying were as formerly described. This SA layered CNF films will be referred to as CNF-SA.
A third variety of CNF films was prepared from these later ones (CNF-SA) as follows: a small amount of the produced modified PCC powder was gently spread on top of the SA layer of the CNF-SA dried films with a fine bristle brush, just enough to cover the surface, in a layer-by-layer assembling (CNF-LBL). After this, the films were subjected to a heat treatment in an oven at either 68 °C or 105 °C for 15 min to improve adhesion between layers. Similar heat treatment was performed on some CNF-SA films.
Structural and physical analysis of CNF films
All samples were kept in standard atmosphere for conditioning (23 °C and 50% of relative humidity) for at least 24 h before testing (ISO 187:1990).
The basis weight of the films was calculated according to ISO 536:2012 and their thickness was measured with a micrometer (Adamel & Lhomargy, M120 series, New York, USA). The arithmetic average of four measurements was used.
The apparent density of the films was determined from the basis weight and thickness.
The corresponding porosity of the films was estimated according to Eq. (3).
where ρfilm is the apparent density of the film, g/cm3 and ρcellulose is the density of crystalline cellulose, which is assumed to be 1.6 g/cm3.
The surface roughness of the produced uncoated CNF and coated CNF films was also assessed using a Bekk smoothness measuring equipment (Messmer Büchel, model K533, Nederlands), in accordance with the Tappi 479 om-91 standard.
The tensile strength, elongation at break and Young’s modulus were determined using a universal tensile testing equipment (Thwing-Albert Instrument Co., EJA series, Philadelphia, USA) with a load cell of 100 N and a constant rate of elongation (20 mm min−1), according to ISO 1924-2:2008. The distance between grips was 50 mm. To avoid slippage at the sample holders, small pieces of paper tape were attached to the edges of the samples.
The dry zero-span tensile tests were performed with a Pulmac tester (Pulmac, TS-100 troubleshooter, Vermont, USA), according to ISO 15361:2000. Static bending stiffness was performed by a bending tester (Lorentzen & Weltre, Sweeden) at an angle of 25 degrees and a 50 mm distance, according to an adaptation made from ISO 5628:2012. For all tests, at least four representative films have been tested and the arithmetic average values are presented.
The optical properties of the films (brightness (ISO 2470-1:2009) and transparency) were determined through spectrophotometry (Technidyne Corp., Color Touch® 2, France).
Barrier properties
The water vapor transmission rate (WVTR) was determined for the produced films according to Tappi 448 om-09. Home customed containers were used, ensuring constant water vapor partial pressures in both sides of the films throughout the essays. The interior of the containers had a given amount of anhydrous granular calcium chloride that ensured zero water vapor partial pressure inside the recipients. The other side of the films was in contact with the standard conditions of temperature and humidity of the laboratory (23 °C and 50% relative humidity). Regarding the coated films (CNF-SA and CNF-LBL) the coated side was facing upward, in contact with the standard conditions. The amount of water vapor that diffused through the films was accounted by the mass increase in the whole set. The whole sets were periodically weighted for as long as 144 h and the mass gain was used to determine the WVTR (g/(m2 day)) of each sample, according to Eq. (4).
where S corresponds to the slope of a liner regression that best fits the plot of weight gain versus testing time (g/days) and A is the area of the films (m2).
The water vapor permeability (WVP) was calculated according to Eq. (5).
where ΔP is the water vapor pressure difference between the two sides of the films and e is the films thickness. Two replicates were performed for each sample.
The oxygen transmission rate (OTR) of the films was measured according to ASTM F1927:2014, using the Ox-Tran®Model 2/21 system (Mocon, Minnesota, USA). The upper limit of the device measuring range was 9570 cm3/(m2 day). The measurements were performed in triplicate at 23 °C and 50% relative humidity. The oxygen permeability (OP) was calculated in a similar manner as the WVP.
Contact angle measurements
Static contact angles (CA) with deionized water were measured with an optical contact angle measuring and contour analysis system (OCAH 200, Dataphysics Instruments, Filderstadt, Germany). The static CA measurements were performed with 4 μL water droplets placed on top of cut out strips of all the produced films, using the sessile drop method. The dynamic (advancing and receding) contact angles (ARCA) were measured by executing consecutive cycles of addition/depletion of 4 μL of water on the 4 μL water droplets already placed on the surface of the samples, using the needle-in-drop method. For every sample, at least 4 measurements were taken, choosing a different spot for each measurement, and the arithmetic average is presented. All measurements were performed at 22 °C and under a relative humidity of 50%.
Statistical analysis
The experimental data were subjected to statistical analysis using SPSS 28 software (SPSS Inc., Chicago, USA) and were examined using analysis of variance (ANOVA). Tukey’s test was used to verify the significant differences among the values at p < 0.05.
Results and discussion
Morphological characterization of the fibrils
The morphology of the fibrils was investigated through SEM and TEM imaging in order to capture the micro- and nano-scale elements. Figure 1 gives an overview of the morphological characteristics of the produced CNFs.
Although it is possible to observe microscale elements (Fig. 1a), the material has been disintegrated into nanofibrils to a considerable extent (Fig. 1b). In fact, a centrifugation method described elsewhere [29] was carried out in order to roughly quantify the relative proportions of micro- and nano-elements in the CNF suspension, and the experimental results showed that nearly 67% of the material has sedimented, indicating that at least 33% of the material is, in fact, nanofibrils. Moreover, the image analysis of the CNF suspensions enabled to determine that about 40% of the material has a length higher than 0.2 mm. The whole material was used to produce the films.
Physicochemical characterization of the fibrils
The determined limiting viscosity of the pretreated fibrils was 154 mL/g, which corresponds to a calculated average DP of 367. This indicates that the resulting material from the TEMPO mediated oxidation had far lower DP than that of the original cellulose used as a starting material (DP of 1429). This result is in good agreement with what has been reported by other authors for TEMPO/NaBr/NaClO oxidation systems [30, 31].
The resulting average carboxyl groups content for the produced TEMPO-oxidized CNFs was 997 μmol/g. Both DP and carboxyl groups content were in good agreement with the results reported by other authors for similar pretreatments [32]. The high carboxyl group content of TEMPO-oxidized cellulose fibrils helps in fiber wall disintegration by loosening the adhesion between microfibrils by electrostatic repulsions and improving aqueous dispersions stability [33]. Considering the substantial carboxyl group content and the relatively low content of nanoelements, it would have been possible to increase this value by performing one or two additional steps in the homogenizer, but probably at the expenses of increasing the film forming time.
Mechanical, optical and barrier properties of the CNF films
The average mechanical and optical-structural properties of all the produced CNF films are presented in Table 1.
Regarding the film formation process, it should be emphasized that the whole CNF suspension was used and that no substantial amount of material was lost during the filtration process (with a 0.65 μm pore size nitrocellulose membrane), since the average obtained basis weight for the CNF films (Table 1) was very close to the target basis weight (55 g/m2). In addition, smooth and translucent CNF films were produced (Fig. 2), in general without the often-reported issue of wrinkling [34]. The produced films were let to air-dry in standard conditions of temperature and humidity, keeping appropriate tension only on the outer edges of the material with metallic rings.
Both apparent density and tensile index of the CNF films measured in this work (Table 1) are in general comparable with the literature results [35,36,37]. For TEMPO-oxidized kraft pulps, Fukuzumi et al. reported tensile strength between 222 and 233 MPa, which taking in account the density can be converted in tensile index between 153 and 160 N m/g [35]. These values are higher than those of the present work, but the film density is also much higher (around 1.45 g/cm3) and the authors removed the non-fibrillated and partially fibrillated materials prior to the film formation. Kumar et al., using TEMPO oxidized eucalyptus and the integral material, reported values in the same range of the present work [38]. Wang et al., using bleached Eucalyptus pulp endoglucanase pretreated, reported values on the same order of magnitude of those presented in Table 1 [36]. The Young modulus values reported in Table 1 range from 5.2 to 5.9 GPa, which are similar to those reported by Fukuzumi et al. [35] for TEMPO-oxidized cellulose (6.2 GPa), but drastically lower than the values reported by Henriksson et al. for cellulose nanopapers produced from softwood dissolving pulp after enzymatic pretreatment with DPs ranging from 410 to 1100, although these authors have dried the nanopapers at 55 °C under a 10 kPa pressure, which certainly played an important role in the mechanical performance [27]. Even for a cellulose with a DP of 410 and a nanopaper porosity of 28%, which are close to our own results, these authors reported a Young’s modulus of 13.7 GPa and overall superior mechanical performance that can probably be attributed to more favorable interfibrillar adhesion characteristics and more homogeneously distributed defects. Regarding the Young modulus, it should be emphasized the contribution of elongation on the result. The films from the present work have a strain at break around 5%, whereas other exhibit lower values and therefore the higher Young’s modulus reported by other authors is not unexpected [38].
The comparison of the values of the tensile index (measured at a span of 50 mm) and the corresponding values for zero-span tensile index enabled to conclude that the major part of the microfibril resistance was retained in the film, suggesting a film relatively free of weak points.
The apparent density slightly decreased with the layer of SA, but consistently increased with the heat treatment (Table 1), which was expected, considering the increased mobility and rearrangement of the SA molecules. This densification process was consistently slightly more pronounced on the samples treated at higher temperature (for the same amount of time). Despite the densification process, the tensile index and overall mechanical performance suffered no major alterations with the heat treatments of any of the films, which makes sense if we acknowledge the CNF layer is the main contributor for the mechanical resistance.
The surface treatment had a significant effect on the films’ transparency: it markedly decreases with the LBL assembling as well as with the SA layer. This is in line with the measured light scattering coefficient and with the dimension and particulate nature of the PCC, which is usually incorporated in office paper during production process to provide opacity [39]. Interestingly, the heat treatment of the coated films enabled to fully recover the transparency of the SA coated films. The effect of the heat treatment on the apparent density may suggest that at least a part of the SA migrates into the pores of the neat CNF films, decreasing the non-contacting interfaces of the CNF elements and therefore decreasing the light scattering coefficient of the material. However, the strong increase in the scattering coefficient from 5.0 to 12.5 cm2/g corroborates the high light scattering power of the SA layer deposited on the CNF films. Actually, as it will be evidenced later on (Fig. 5c), the surface irregularities of the SA layer are under 1 μm, meaning they are in the same order of magnitude of visible light wavelength (400–700 nm), and scattering it. For the CNF-LBL films, an important transparency recovery occurs with heat treatment, but the light scattering power of the PCC in the surface and also possibly inside the film is preserved and the transparency remains lower than that of the neat CNF films (Fig. 3).
Water vapor and oxygen barrier properties
Figure 4 shows the obtained results for the accumulated water vapor transferred over time through the different produced CNF films, which enabled to estimate the WVTR (Table 2).
The neat CNF films exhibit WVTRs comparable with the values reported for similar films produced from TEMPO-oxidized pulps [40] and higher than those reported for enzymatically treated pulps and bacterial cellulose films (27.1 g/(m2 day); global porosity = 29.6%) [41]. Migration of molecules between two adjacent volumes separated by a thin film occurs in three basic steps: adsorption of the molecule on the film’s surface, diffusion through the film and exit of the diffusing molecule by desorbing from the surface [42]. As any other mass transfer process by diffusion, the WVTR will be proportional to the effective water vapor diffusion and the driving force. In turn, the effective water vapor diffusion depends on the water vapor diffusion in air, but also on porosity and pore tortuosity. In addition, if the pore size is of the same magnitude of the free average path of molecules, Knudsen diffusion takes place. Thus, it is plausible to think that WVTR would escalate with the increase of the fibril’s carboxyl group content, due to increased affinity to water on the water vapor exposed film side (the other side is water free; therefore, the driving force increases).
Costa et al. have reported extremely low WVTR values (7.53 g m−2 day−1) for bacterial cellulose films after hot calendering treatment, with a decrease in global porosity from 29.6 to 5.4% [41].
As it is observable in Fig. 4 and Table 2, the surface treatment with SA has a small positive impact on the WVTR. More importantly, the heat treatment of the CNF-SA films has a tremendous positive impact on the WVTR. The WVTR decreases from 92 to 38 g m−2 day−1, when the sample was submitted to a heat treatment during 15 min at 68 °C. This temperature treatment certainly provides some fluidity to the SA layer (the melting point of SA is 69.3 °C). Furthermore, the increase in heat temperature from 68 to 105 °C slightly decreased the WVTR of the films, which validates the supposition that the SA layer melts during heat treatment and the SA molecules fill the interstitial pore space in the fiber network as they gain more mobility with increasing temperature.
This migration of the SA with temperature to the internal pores of the CNF films is also consistent with the decrease in the light scattering coefficient of the heat-treated CNF-SA films (Table 1).
CNF-LBL films showed even lower WVTR relatively to the CNF-SA films, although in this case the effect of increasing heat treatment is not so evident.
Spence et al. reported that coating microfibrillated cellulose (MFC) composite films with beeswax or paraffin decreases the WVTR and hypothesized that this could be due to surface pore closure and filling of the pore network [43]. On another study, paraffin wax (8.6 wt%) was incorporated on pretreated bleached softwood and resulted in a decrease of the WVTR of the produced films, in agreement with a pore filling mechanism [44]. However, the decrease in global porosity alone (from around 30 to 25%) is not enough to justify the drastic decrease in the WVTR and the decrease in surface porosity may be the key factor.
Oxygen transmission rate
The oxygen transmission rate values measured in the neat CNF films are, in general, consistently higher than those reported in the literature [45, 46]. Syverud and Stenius, working with mechanically fibrillated cellulose, have reported values of OP in the range 3.52–5.03 cm3 µm/(m2 day kPa) [47]. For CNF produced after TEMPO oxidation, the reported values are even much lower [6, 35, 45]. Österberg et al. have reported values of 0.6 cm3 µm/(m2 day kPa). In the present work, the oxygen permeability is around 26.4 cm3 µm/(m2 day kPa) and present a very high standard deviation [6]. The morphological evaluation of the CNF suspension by image analysis enabled to detect a significant fraction of fibrous material in the class length of > 0.2 mm (see also Fig. 1). The presence of this significant amount of fiber fragments and the reported low crystallinity index of the CNFs pretreated with TEMPO are the main possible reasons behind the observed higher than expected OP values. Padberg et al. have shown that TEMPO oxidized pulp exhibits lower crystallinity index, which induces higher oxygen permeability [48]. In addition, the high standard deviation suggests the existence of some nonuniformities in the film at nanoscale levels. The effect of the LBL coating on the OTR and OP is notorious. The OP decreased from 26.4 to 2.6 cm3 µm/(m2 day kPa) after surface modification with SA and modified PCC. The closure of superficial pores by the stearic acid and the PCC mineral is probably the reasons behind these results. Unfortunately, the OTR was not measured in the stearic acid coated samples and therefore the relative contribution of the SA and mineral coating cannot be disclosed. In addition, it should be emphasized that OTR of these coated CNF films compare favorably with the synthetic ones (p.e. Ethylene vinyl alcohol: 3–5 cm3/(m2 day) [49].
Figure 5 depicts the microscopic appearance of an uncoated CNF film and SA coated CNF films, before and after heat treatment at both tested temperatures.
As it is possible to see in Fig. 5, unlike an uncoated CNF film (Fig. 5a) the SA layers without any heat treatment present the engraved marks from the cloths with which they were in contact during the pressing stage (Fig. 5b). The perceptible surface smoothness of the SA layer after heat treatment at 68 °C (Fig. 5d, e) and 105 °C (Fig. 5f, g) is an indicator of a melting process. It is not visually perceptible any major difference between the SA surfaces treated at the two different temperatures (Fig. 5d, f), but the nanoscale irregularities are clear on the surface of the air-dried CNF-SA (c), which may also contribute to the observed light scattering (Table 1).
To modify the surface roughness at a micro- and nano-scale as a way to enhance hydrophobicity, a modified PCC powder was applied on the CNF-SA films.
The modified PCC powder could be easily spread on the top of previously SA coated CNF films, producing CNF-LBL films. Although water droplets placed on these films could smoothly slide across their surface, clearly exhibiting lotus effect (Fig. 3b), the PCC powder layer was just loosely attached to the SA layer and could easily be removed with touch or friction. In order to overcome this critical issue and strengthen the bonding between the SA layer and the modified PCC heat treatment was performed on the films. After a preliminary study, we concluded that 68 °C was the minimum temperature at which the modified PCC powder was able to strongly adhere to the SA surface, in which case the partially melted SA layer acted as a glue between the modified PCC powder layer and the CNF film. On the one hand, a small part of the SA layer migrated into the pores of the neat CNF film, providing mechanical strength between the SA layer and the CNFs; on the other hand, a small portion of the SA molecules on the surface of the modified PCC merged on the underlying SA layer, providing adhesion to the PCC particles. The PCC layer on the top of the produced heat-treated CNF-LBL films (68 and 105 °C) was resistance to touch and friction, maintaining their hydrophobic properties after handling or rubbing with a finger across their surface.
Contact angle measurements
The hydrophilic nature of untreated CNF films is a well-known feature that arises from their high hydroxyl and carboxyl content which may in turn lead to low resistance to water [35]. The most common method for determining the hydrophilic/ hydrophobic nature of a solid is the measurement of its contact angle (CA) with water. The static CA is specific to any given system and can be defined as the angle at which a droplet of probing fluid (water, in this case) meets a given solid, being determined by the interactions across the three-phase line. Generally, the CA is lower than 90° for hydrophilic materials, while hydrophobic ones display a CA higher than 90° [11]. A particular case of hydrophobicity is superhydrophobicity which is characterized by a static contact angle above 150° and also a very small sliding angle, typically lower than 5° [50].
Figure 6 shows the variation of CA over time of a water droplet placed on a neat CNF film and an CNF-SA film with no heat treatment.
Predictably, the neat CNF films were hydrophilic with an initial CA of around 45°. The contact angle decreased with time as a result of partial penetration of water into the film, coming down to 35° after 3 s (Fig. 6). The CA of CNF films can differ according to many different factors, not only chemical composition, but the fibrillation degree of the obtained fibrils as well: a more fibrillated material leads to a more compact and less porous surface, which consequently increases the observed CA [51].
The CA of the neat CNF films is in good agreement with Fukuzumi et al., who reported an initial CA of 47° on an CNF film prepared from TEMPO-oxidized softwood cellulose with a carboxyl group content of 1300–1600 μmol/g, and likewise described the same phenomenon of CA decreasing over time due to partial water penetration [35].
The CNF-SA film turned out to be hydrophobic, since their measured CA was around 122°. This result resembles that reported by Österberg et al. where CNF films were surface coated using a commercial paraffin wax and the CA increased from 40 to 110° for uncoated and wax coated films, respectively [6].
While the CA of the neat CNF films decreased over time due to swelling, the CA for CNF-SA remained constant for the whole measuring time (3 s), since there was no water penetration into the film due to decreased wettability.
The CA of all the produced films was determined and the results are presented in Table 3. The reported values of CA correspond to the measurement at 3 s to avoid initial instability due to the water droplet wobbling. For visual comparison, the snapshots of the correspondent water droplets at 3 s measurements are also shown.
As presented in Table 3, the application of a SA layer on the surface of the films with no heat treatment was sufficient for providing them with hydrophobicity, attaining a CA of 122°. While the layer of SA successfully hydrophobized the simply air-dried films, heat treatment at 68 °C and 105 °C decreased their CA to 96° and 92°, respectively (Table 3), possibly due to melting of the SA, causing the surface to be smoother (Fig. 5). Forsman et al. applied a carnauba wax dispersion on CNF films using a layer-by-layer coating method and have also performed heat treatment at 103 °C in order to investigate melting of the wax particles and its effect on the coating. They came to the conclusion that the melting of the wax smooths the surface of the films, causing a slight decrease in CA when compared to the untreated films, which is in good agreement with our result [4].
The addition of modified PCC particles, combined with a heat treatment at 68 °C for adhesion, had a notorious positive effect, increasing the CA from 96 to 140°, probably due to the increase in roughness. In order to assess the surface roughness (as an opposite of smoothness) of the uncoated and coated films, Bekk smoothness tests were performed in triplicate on the surface of all produced films and the mean values are reported in Table 4. While the SA coating and especially the LBL coating with no heat treatment drastically increased the surface roughness (Bekk smoothness decreased from 268 to 34 s), heat treatment was able to restore some smoothness to those surfaces, particularly for the films treated at 105 °C.
Figure 7 shows the microscopic surface appearance and a cross section of an CNF-LBL film, after heat treatment at 68 °C.
In Fig. 7 it is possible to identify micro- and nano-sized particles of modified PCC powder spread across the surface of the heat-treated CNF-LBL film (Fig. 7a, b). Despite the melting process of the SA layer acting as a binder between the CNF film and the modified PCC, the powder did not completely sink into the SA layer, generating roughness on its surface (Fig. 7c). The PCC particles uniformly covered all the surface of the film (Fig. 7b), providing hydrophobicity since it had a measured CA of 140° (Table 3), although the literature reports that only partial nanoparticle coverage can be enough to significantly change the wetting properties of a surface [4, 52]. Furthermore, while surfaces with high macroscale roughness may cause inconsistent contact angle measurements, the positive effect of micro- and nano-scale roughness on hydrophobicity has been reported by many authors [53,54,55].
The cross section of an CNF-LBL film, shown in Fig. 7c with the modified PCC powder facing upward, enabled to perceive that the layered film is actually quite compact and uniform, with no distinguishable layered fractions, which certainly contributes to the physical integrity of the whole set.
According to Table 3, the increase in temperature in the heat treatment from 68 to 105 °C slightly decreased the CA of the CNF-LBL films from 140 to 135°, probably because the SA layer became melted enough for some modified PCC particles to completely sink in, slightly decreasing the overall surface’s roughness (Table 4). Even though the SA layer only constitutes 5% of the total weight of the layered film, this result shows that adjustments in the amount of SA used to coat the CNF films, eventually reducing the thickness of the SA layer, might come as advantageous.
Another important parameter when researching hydrophobic surfaces is their CA hysteresis, i.e., the difference between the advancing CA and the receding CA [11, 56]. ARCA measurements were performed as follows: the advancing CA was measured by gradually increasing the volume of a probe droplet until the three-phase (solid–liquid–gas) contact line begins to expand, at which point a maximum CA was reached. Reciprocally, the receding CA was measured by gradually decreasing the volume of a sessile droplet until the contact line began to shrink, at which point the receding CA can be obtained [57].
For a super-hydrophobic surface for instance, apart from having a static CA above 150 °C, the water droplet shall not stick to the surface of the sample, having thus low CA hysteresis [11, 19].
ARCA measurements were performed on CNF-SA and CNF-LBL films with and without heat treatment.
The obtained CA hystereses for the CNF films are plotted in Fig. 8, as ARCA cycles (succeeding increase and decrease of the probe droplet’s volume as a function of time).
It is worth mentioning that the initial CAs registered in Fig. 8 are somewhat lower than those previously reported as static CAs for the respective films in Table 3. The reason for this is related to the different CA measuring techniques: while a typical static CA measurement is carried out using a sessile drop method, where the probe droplet is placed on top of the sample and the needle is removed before the measurement, an ARCA cycle involves CA measurements performed using a needle-in-drop method, which is a requirement for increasing and decreasing its volume. The decrease in the CA arises as a consequence of the additional adhesion between the water and the needle, which slightly changes the water droplet geometry, leading to lower measured contact angles. From the results, it is evident that the CNF-LBL samples exhibit higher contact angles and lower hysteresis values than the CNF-SA samples, highlighting the role of the micro/nanoscale roughness on the hydrophobic and super hydrophobic behavior.
Hu and Deng used a comparable approach to achieve a superhydrophobic layer through chemically modifying PCC particles in water with oleic acid. They studied the CA hysteresis of the modified material and reported a CA hysteresis of 2.5° for an oleic acid content of 1.09 wt% and a CA hysteresis of nearly zero for an optimum oleic acid content of 2.51 wt%, with a corresponding advancing water CA of 164° [58]. These results are not far from those achieved in the present work, where an CNF-LBL film with no heat treatment attained a CA hysteresis of 3.1° (149.5–146.4°) and a static CA of 150°. Even after the heat treatment at 68 °C, a CA hysteresis of 5° (131–126°) was reached, with a static CA of 140°. Furthermore, while Hu and Deng performed CA measurements on the modified PCC particles using double-sided adhesive tape as the substrate, in the present work, through a simple heat treatment, we were able to permanently adhere modified PCC particles on CNF films surface, using an intermediate layer of stearic acid as a binder [58].
Conclusions
Smooth and translucent CNF films were produced through vacuum filtration using 0.65 μm pore size nitrocellulose membranes as filtration media, with no substantial loss of material during the filtration process.
Both apparent density and tensile index of the produced CNF films, varying from 1.10 and 1.24 g/cm3, and from 101.6 and 111.7 Nm/g, respectively, are in general comparable with the literature results. The overall mechanical performance of the films suffered no major alterations after surface treatment with SA and modified PCC and with posterior heat treatments.
Concerning barrier properties, heat treatments decreased the WVTR of SA layered CNF films from 91.9 down to 31.6 g m−2 day−1, probably due to SA melting, filling the interstitial pore space in the fiber network. The closure of superficial pores by SA and modified PCC also caused a drastic decrease in the oxygen permeability from 26.4 to 2.6 cm3 µm/(m2 day kPa).
Surface coating CNF films with a SA layer rendered them hydrophobic, increasing the water CA from 45 to 122°. Heat treatments at 68 °C and 105 °C decreased the CA to 96° and 92°, respectively, due to melting of the SA layer, causing the surface to be smoother. On the other hand, the addition of modified PCC increased the CA from 96 to 140° with a heat treatment of 68 °C, and to 150 °C, with no heat treatment, most likely due to nanoscale roughness provided by the modified PCC particles.
The heat treatment was a key stage in the process, enabling the adherence of the modified PCC particles onto the stearic acid layer and the later to the CNF network, providing a quasi-superhydrophobic film with good resistance to handling and rubbing, using an easy and green process.
References
Jenck JF, Agterberg F, Droescher MJ (2004) Products and processes for a sustainable chemical industry: a review of achievements and prospects. Green Chem 6(11):544
Abdul Khalil HPS, Bhat AH, Ireana Yusra AF (2012) Green composites from sustainable cellulose nanofibrils: a review. Carbohyd Polym 87(2):963–979
Ivanković A, Dronjić A, Bevanda AM, Talić S (2017) Review of 12 principles of green chemistry in practice. Int J Sustain Green Energy 6(3):39–48
Forsman N, Lozhechnikova A, Khakalo A, Johansson L-S, Vartiainen J, Österberg M (2017) Layer-by-layer assembled hydrophobic coatings for cellulose nanofibril films and textiles, made of polylysine and natural wax particles. Carbohyd Polym 173:392–402
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohyd Polym 79(4):1086–1093
Österberg M, Vartiainen J, Lucenius J, Hippi U, Seppälä J, Serimaa R, Laine J (2013) A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl Mater Interfaces 5(11):4640–4647
Kalia S, Boufi S, Celli A, Kango S (2014) Nanofibrillated cellulose: surface modification and potential applications. Colloid Polym Sci 292(1):5–31
Stark NM (2016) Opportunities for cellulose nanomaterials in packaging films: a review and future trends. J Renew Mater 4(5):313–326
Gonçalves G, Marques PAAP, Trindade T, Neto CP, Gandini A (2008) Superhydrophobic cellulose nanocomposites. J Colloid Interface Sci 324(1–2):42–46
Jonoobi M, Harun J, Mathew AP, Hussein MZB, Oksman K (2010) Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 17(2):299–307
Song J, Rojas OJ (2013) Paper chemistry: approaching super-hydrophobicity from cellulosic materials: a review. Nord Pulp Pap Res J 28(2):216–238
Rastogi V, Stanssens D, Samyn P (2014) Mechanism for tuning the hydrophobicity of microfibrillated cellulose films by controlled thermal release of encapsulated wax. Materials 7(11):7196–7216
Sehaqui H, Zimmermann T, Tingaut P (2014) Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 21(1):367–382
Yuan Z, Wen Y (2018) Enhancement of hydrophobicity of nanofibrillated cellulose through grafting of alkyl ketene dimer. Cellulose 25(12):6863–6871
Rol F, Belgacem MN, Gandini A, Bras J (2019) Recent advances in surface-modified cellulose nanofibrils. Prog Polym Sci 88:241–264
Kalia S, Thakur K, Celli A, Kiechel MA, Schauer CL (2013) Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: a review. J Environ Chem Eng 1(3):97–112
Liimatainen H, Ezekiel N, Sliz R, Ohenoja K, Sirviö JA, Berglund L et al (2013) High-strength nanocellulose-talc hybrid barrier films. ACS Appl Mater Interfaces 5(24):13412–13418
Samyn P (2013) Wetting and hydrophobic modification of cellulose surfaces for paper applications. J Mater Sci 48(19):6455–6498. https://doi.org/10.1007/s10853-013-7519-y
Mertaniemi H, Laukkanen A, Teirfolk J-E, Ikkala O, Ras RHA (2012) Functionalized porous microparticles of nanofibrillated cellulose for biomimetic hierarchically structured superhydrophobic surfaces. RSC Adv 2(7):2882
Huang J, Lyu S, Fu F, Chang H, Wang S (2016) Preparation of superhydrophobic coating with excellent abrasion resistance and durability using nanofibrillated cellulose. RSC Adv 6(108):106194–106200
Arbatan T, Fang X, Shen W (2011) Superhydrophobic and oleophilic calcium carbonate powder as a selective oil sorbent with potential use in oil spill clean-ups. Chem Eng J 166(2):787–791
Hu Z, Zen X, Gong J, Deng Y (2009) Water resistance improvement of paper by superhydrophobic modification with microsized CaCO3 and fatty acid coating. Colloids Surf, A 351(1–3):65–70
El-Sheikh SM, Barhoum A, El-Sherbiny S, Morsy F, El-Midany AA-H, Rahier H (2019) Preparation of superhydrophobic nanocalcite crystals using Box-Behnken design. Arab J Chem 12(7):1479–1486
Wang C, Sheng Y, Zhao X, Pan Y, Hari-Bala WZ (2006) Synthesis of hydrophobic CaCO3 nanoparticles. Mater Lett 60(6):854–857
Wang C, Piao C, Zhai X, Hickman FN, Li J (2010) Synthesis and characterization of hydrophobic calcium carbonate particles via a dodecanoic acid inducing process. Powder Technol 198(1):131–134
Xu M, Dai H, Sun X, Wang S, Wu W (2012) Influence of buffer solution on tempo-mediated oxidation. BioResources 7(2):1633–1642. https://doi.org/10.15376/biores.7.2.1633-1642
Henriksson M, Berglund LA, Isaksson P, Lindström T, Nishino T (2008) Cellulose nanopaper structures of high toughness. Biomacromolecules 9(6):1579–1585
Lozhechnikova A, Bellanger H, Michen B, Burgert I, Österberg M (2017) Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl Surf Sci 396:1273–1281
Costa VLD, Costa AP, Simões RMS (2019) Nanofibrillated cellulose rheology: effects of morphology, ethanol/acetone addition and high NaCl concentration. BioResources 14(4):7636–7654
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohyd Polym 84(3):975–983
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85
Shinoda R, Saito T, Okita Y, Isogai A (2012) Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules 13(3):842–849
Saito T, Nishiyama Y, Putaux J-L, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7(6):1687–1691
Sehaqui H, Liu A, Zhou Q, Berglund LA (2010) Fast preparation procedure for large, flat cellulose and cellulose/inorganic nanopaper structures. Biomacromolecules 11(9):2195–2198
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 10(1):162–165
Wang W, Sabo RC, Mozuch MD, Kersten P, Zhu JY, Jin Y (2015) Physical and mechanical properties of cellulose nanofibril films from bleached eucalyptus pulp by endoglucanase treatment and microfluidization. J Polym Environ 23(4):551–558
Lindström T (2017) Aspects on nanofibrillated cellulose (NFC) processing, rheology and NFC-film properties. Curr Opin Colloid Interface Sci 29:68–75
Kumar V, Bollström R, Yang A, Chen Q, Chen G, Salminen P, Bousfield D, Toivakka M (2014) Comparison of nano- and microfibrillated cellulose films. Cellulose 21:3443–3456
Shen J, Song Z, Qian X, Liu W (2009) Modification of papermaking grade fillers: a brief review. BioResources 4(3):1190–1209
Bedane AH, Eić M, Farmahini-Farahani M, Xiao H (2015) Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J Membr Sci 493:46–57
Costa VLD, Costa AP, Amaral ME, Oliveira C, Gama M, Dourado F, Simões RM (2016) Effect of hot calendering on physical properties and water vapor transfer resistance of bacterial cellulose films. J Mater Sci 51(21):9562–9572 https://doi.org/10.1007/s10853-016-0112-4
Nair SS, Zhu J, Deng Y, Ragausks AJ (2014) High performance green barriers based on nanocellulose. Sustain Chem Process 2(23):1–7. https://doi.org/10.1186/s40508-014-0023-0
Spence K, Venditti R, Rojas O, Pawlak J, Hubbe M (2011) Water vapor barrier properties of coated and filled microfibrillated cellulose composite films. BioResources 6(4):4370–4388
Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2010) The effect of chemical composition on microfibrillar cellulose films from wood pulps: water interactions and physical properties for packaging applications. Cellulose 17(4):835–848
Aulin C, Gällstedt M, Lindström T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Lavoine N, Desloges I, Dufresne A, Bras J (2012) Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohyd Polym 90(2):735–764
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16(1):75–85
Padberg J, Bauer W, Gliese T (2016) The influence of fibrillation on the oxygen barrier properties of films from microfibrillated cellulose. Nord Pulp Pap Res J 31(4):548–560
Kim D, Kwon H, Seo J (2013) EVOH nanocomposite films with enhanced barrier properties under high humidity conditions. Polym Compos 53(4):644–654
Lv C, Yang C, Hao P, He F, Zheng Q (2010) Sliding of water droplets on microstructured hydrophobic surfaces. Langmuir 26(11):8704–8708
Sharma S, Nair SS, Zhang Z, Ragauskas AJ, Deng Y (2015) Characterization of micro fibrillation process of cellulose and mercerized cellulose pulp. RSC Adv 5(77):63111–63122
Dong L, Nypelö T, Österberg M, Laine J, Alava M (2010) Modifying the wettability of surfaces by nanoparticles: experiments and modeling using the Wenzel law. Langmuir 26(18):14563–14566
Cao L, Hu H-H, Gao D (2007) Design and fabrication of micro-textures for inducing a superhydrophobic behavior on hydrophilic materials. Langmuir 23(8):4310–4314
Bhushan B, Koch K, Jung YC (2008) Biomimetic hierarchical structure for self-cleaning. Appl Phys Lett 93(9):093101
Rodionova G, Eriksen Ø, Gregersen Ø (2012) TEMPO-oxidized cellulose nanofiber films: effect of surface morphology on water resistance. Cellulose 19(4):1115–1123
Kusumaatmaja H, Yeomans JM (2007) Modeling contact angle hysteresis on chemically patterned and superhydrophobic surfaces. Langmuir 23(11):6019–6032
Ras RHA, Tian X, Bayer IS (2017) Superhydrophobic and superoleophobic nanostructured cellulose and cellulose composites. In: Kargarzadeh H, Ahmad I, Thomas S, Dufresne A (eds) Handbook of nanocellulose and cellulose nanocomposites. Wiley, Weinheim, pp 731–760
Hu Z, Deng Y (2010) Superhydrophobic surface fabricated from fatty acid-modified precipitated calcium carbonate. Ind Eng Chem Res 49(12):5625–5630
Acknowledgements
The research undertaken for this paper was performed under the UBI-Celtejo cooperation protocol. The authors are very grateful for the support given by research unit Fiber Materials and Environmental Technologies (FibEnTech-UBI), on the extent of the project reference UIDB/00195/2020, funded by the Fundação para a Ciência e a Tecnologia, IP/MCTES through national funds (PIDDAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costa, V.L.D., Simões, R.M.S. Hydrophobicity improvement of cellulose nanofibrils films by stearic acid and modified precipitated calcium carbonate coating. J Mater Sci 57, 11443–11459 (2022). https://doi.org/10.1007/s10853-022-07249-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07249-x