Abstract
Classical sessile drop experiment was performed for intensive microstructure and phase composition studies of the reaction product region (RPR) formed because of high temperature interaction between the aluminum–copper alloy (Al—16.7 at.% Cu) and SiO2 (amorphous) substrate. The experiment was performed in vacuum at 1173 K for 2 hour contact time. Scanning and transmission electron microscopy techniques were applied to reveal the details of the complex microstructure of reactively formed product zone at the drop/substrate interface. Three regions of different structure and phase composition were well distinguished in the RPR: the first layer was composed of large-faced Al2O3 crystals of alpha type surrounded by the Al2Cu metallic channels, the second one had the same phase composition but different morphology of the alpha alumina and with silicon as an extra component (dissolved within these phases and as precipitates). The third area revealed very fine-grained (100–200 nm) microstructure, in which Al2Cu and Si grains were embedded in orthorhombic δ-Al2O3 matrix. Moreover, the presence of the deformation twins in silicon, twinned on (1–11) plane was related to large strains present in the area close to the SiO2 substrate and coming from the volumetric mismatch of SiO2 and the freshly formed Al2O3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composite materials based on Al–Al2O3 system are characterized by the unique set of properties such as high hardness, good resistance to abrasion, low density, and thermal and electrical conductivities, accompanied by a low price. The displacement reaction between Al and SiO2 precursor provides the basis for the in situ formation of Al2O3 in C4-type composites (co-continuous ceramic composites) composed of mutually interconnected ceramic (Al2O3) and metallic (Al-based) skeletons [1–4].
For practical reasons, alloying aluminum may be useful to decrease the processing temperature. However, the alloying elements may change both the phase composition and the morphology of reactively formed phases, thus affecting the properties of the final product. Therefore, information on the effect of alloying elements on the type and morphology of reactively formed alumina is of practical importance.
The study by Sobczak et al. [5] using optical microscopy (OM) and scanning electron microscopy (SEM) techniques did not show any new reactively formed phases after alloying Al with 16.7 at.% Cu (near eutectic composition with corresponding low melting temperature of 550 K) and subsequent long-term contact with SiO2 at 1273 K. However, the thickness of reaction product region (RPR), as a measure of reactivity in the system, was decreased from 1.2 mm in Al/SiO2 to 0.5 mm in Al16.7Cu/SiO2. Recently, Santhage [6] has reported (after Strange and Breslin [7] and Evarts [8]) the influence of Al–Cu melt composition on the domain sizes of reactively formed alumina in the composites of C4 structure produced by the immersion of dense amorphous silica in a molten Al–Cu bath containing 0–80 at.% Cu. Compared with pure Al, Al–Cu melts exhibited lower linear penetration rates with dramatic reductions in the colony sizes of co-continuous alumina and AlCu and Al2Cu intermetallic phases formed at 1373–1423 K.
Similar effect of alloying Al with Cu was reported also in [9] for Al/mullite system, but it became less pronounced with the increase of temperature, i.e., at 1173 K, the RPR thickness was 1.5 mm for pure Al and 1.1 mm in Al16.7Cu, while at 1273 K, they were 1.8 and 1.6 mm, respectively.
In order to understand the effect of Cu addition on the interaction between Al–Cu melts and SiO2-based precursors, the detailed structural characterization of the reaction products region is needed. Particularly, it is important to identify the type of reactively formed alumina depending on its location in the RPR, which was not done in the above mentioned reports [4–7]. As has already been demonstrated in the previous study [10], at 1273 K, pure Al reduces both the SiO2 and mullite constituents of fly ash to form the co-continuous α-alumina. In the case of Al/MgO system, the redox reaction causes the formation of co-continuous α-alumina and MgAl2O4 phases, depending on testing conditions [11, 12], while in the Al/MgAl2O4 system, the only co-continuous α-alumina is formed [12]. The studies by Wojewoda-Budka et al. [13, 14] using transmission electron microscopy (TEM) revealed that, at the same temperature, the redox reaction in pure Al/ZnO couples, also leading to the formation of the RPR of C4 structure, can be accompanied by the formation of dissimilar alumina types such as alpha, delta, and alumina of unknown type, depending on the nature of ZnO (polycrystalline, single crystal, etc.) [13] as well as on the crystallographic orientation of ZnO single crystal [14].
In this study, the detailed structural characterization of Al16.7Cu/SiO2 couple, produced at industrially important temperature of 1173 K, has been performed using focused ion beam (FIB) technique that allowed cutting out precisely the chosen areas from the reaction product region of the cross-sectioned couple for their careful observations using different TEM techniques.
Experimental
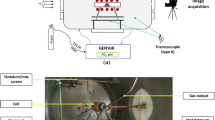
The Al–Cu alloy containing 16.7 at.% Cu (32 wt% Cu) and amorphous silica substrates of 1.5 mm in thickness were used in the experiments. They were ultrasonically cleaned in acetone before the test. The Al16.7Cu/SiO2 couple was produced in the sessile drop test using contact heating at 1173 K for 2 h under dynamic vacuum condition of 9–20 × 10−6 hPa and subsequent cooling at a rate of ~14 K/min [2]. The microstructure of the cross-sectioned couples (Fig. 1a) was first examined using the SEM FEI E-SEM XL30 equipped with the EDAX Genesis energy dispersive X-ray (EDX) spectrometer. This enabled preliminary microstructure characterization and a quantitative point analysis. The thin foils for the TEM examinations were obtained using a FIB technique on FEI Quanta 3D instrument. Finally, the TEM characterizations comprising bright field (BF) images, the selected area electron diffraction (SAED) patterns, and the EDX maps of element distributions were obtained using TECNAI G2 FEG super TWIN (200 kV) microscope equipped with high angle annular dark field (HAADF) detector and integrated with EDX system manufactured by EDAX company.
SEM (BSE) microstructures of the Al16.7Cu/SiO2 cross section: general view of the reaction product region showing also part of the drop and SiO2 (a) with three marked areas of RPR examined at higher magnifications, i.e., the RPR just below the drop (b), in the middle (c), and substrate-side interface (d). The arrows indicate the places of TEM analyses: “FIB cut 1” (c) and “FIB cut 2” (d)
Results
During the 2-h interaction at 1173 K between the Al–Cu alloy and SiO2, the RPR of over 1 mm in thickness was produced inside the SiO2 substrate under the drop. Large cracks were present within the substrate and under the RPR.
Figure 1 represents the backscattered electron image of the sample microstructure where the electrons were applied as a primary beam. The SEM examination of RPR revealed three regions of different structure and phase composition, marked as 1, 2, and 3 in Fig. 1b–d. The first one, formed at the drop side as a layer of about 30 μm in thickness, was composed of coarse dark particles in a bright matrix (Fig. 1b). Occasionally, this layer is extended also within the RPR far from the initial drop/substrate interface (Fig. 1c, d). The second region of bright-gray color in Fig. 1c, d existed in the central part of the RPR as well as at its substrate side. The third region, composed of fine bright-gray and dark-gray phases of irregular shapes, was located between the above two regions (Fig. 1b, c).
EDX analyses identified coarse precipitates as Al2O3 phase surrounded by Al2Cu metallic channels in area 1. Three phases could be distinguished in area 2 at higher magnification: very bright phase with contrast similar to that of Al2Cu, dark-gray phase similar to Al2O3 and light-gray one. Area numbers 2 and 3 with either very fine precipitates or interpenetrating networks the EDX analysis of the individual phases were impossible to be correctly measured in the SEM because of the beam broadening and escape depth of X-rays.
Area 3 (Fig. 1c) was selected and cut using the FIB technique for further detailed TEM characterization to identify particular phases and to determine the crystallography of the reactively formed alumina particles. The TEM analysis of thin foil taken from area 3 (Fig. 2) confirmed typical C4-type microstructure composed of two interpenetrating networks: bigger and brighter ceramic precipitates, compared with the darker metallic channels, surrounding these precipitates.
TEM analysis of RPR in area 3 from Fig. 1c: general view (a) with SAED pattern of bright Al2O3 phase and maps of Al (b), Cu (c), O (d), and Si (e) distribution. Microstructure of the Si precipitate marked with black arrow is presented in (f)
The energy dispersive X-ray analysis together with the SAED allowed the determination of those particles as alumina phase of α-Al2O3 type which was the most thermodynamically stable compared with other aluminium oxides. It was identified as the corundum structure with a hexagonal close-packed arrangement. The morphology of those precipitates differed from the big-faceted alumina crystals of a few to over ten micrometers in diameter present in the vicinity of the drop/RPR interface.
The map of element distribution presented in Fig. 2b–e confirmed that the microstructure was mostly composed of Al2O3 phase interpenetrated with the metallic channels containing copper and aluminum, while silicon was dissolved in both the alumina matrix and in the Al–Cu metallic channels, and also located as separated precipitates (Fig. 2a, e). According to the explanation found in another study [3], Si tends to nucleate at the alumina surface within the Al matrix, as shown in Fig. 2f. Furthermore, TEM/EDX examination has shown the presence of oxygen within the Al–Cu alloy channels (4.6 at.% O, 62.7 at.% Al, 31.8 at.% Cu, and 0.9 at.% Si), thus suggesting a possible formation of either an inverse spinel CuAl2O4 or the CuAlO2 phase. As per the Al–Cu–O phase diagram, the liquid metallic solution is in equilibrium with Al2O3 throughout a wide range of composition as the copper oxides are much less stable. Both CuAl2O4 and CuAlO2 can be formed by eutectoid reaction between Al2O3 and CuO in air (CuAl2O4 above 873 K, CuAlO2 at T > 1273 K), i.e., with unlimited excess of oxygen [15, 16] that is not the case for the testing conditions used in this study. Further detailed TEM examination using SAED patterns (Fig. 3) showed that the metallic channels consisting of Al2Cu precipitates are dispersed in the Al–Cu–Si alloy. They are characterized by the tetragonal crystal structure with the cell parameters of a = 6.067 Å and c = 4.887 Å given in the Ref. [17].
A fine-grained microstructure in the area close to the silica substrate is presented in Fig. 4a–b. The recognition of the composition of fine grains was not an easy issue due to their mutual imposition. The results of chemical point analysis in particular grains could be incorrectly interpreted; for example, the presence of mullite was incorrectly reported by some researchers [18]. The map of the element distribution (Fig. 4c–g) showed the presence of two types of the grains, enriched either in silicon or copper, and surrounded by the matrix containing aluminum and oxygen. Selected area diffraction patterns taken from both types of grains revealed that they were silicon (Fig. 4h) and Al2Cu (Fig. 4i) phases surrounded by the alumina phase (Fig. 4j). Moreover, at least two interesting features were observed. First, the presence of the deformation twins in silicon, twinned on (1–11) plane (Figs. 4b, h), is probably related to the large strains present in the area close to the SiO2 substrate and comes from the volumetric change between SiO2 and Al2O3. Second, selected area diffraction patterns of the alumina (Fig. 4j) definitely excluded the presence of α-Al2O3. Therefore, various metastable alumina samples extensively reported in the study by Levin and Brandon [19] were taken into account, among which the orthorhombic δ-Al2O3 with lattice parameters a = 7.9 Å, b = 15.8 Å, c = 11.85 Å gave the best match.
TEM analysis of RPR in area 2 from Fig. 1d: general view of the fine grained microstructure (a) together with the higher magnification exposing the twinned grains marked with the arrows (b). STEM image (c) and corresponding maps of Al (d), Si (e), Cu (f), and O (g) distribution. Selected area electron diffraction patterns of δ-Al2O3 (h), Al2Cu phase (i) and silicon twinned on (1–11) plane (J)
Discussion
A schematic illustration of the microstructure observations in the cross section of the solidified Al16.7Cu/SiO2 couple is presented in Fig. 5. Compared with the RPR formed inside the SiO2 substrate under pure Al, the RPR in the Al16.7Cu/SiO2 couple has highly heterogeneous structure with unusual distribution of the identified area of dissimilar phase compositions.
The reactively formed α-Al2O3 phase is the main constituent of RPR. Its large dark gray precipitates were interpenetrated with metallic network, as it is typical for ceramic–metal composites of C4 structure (Fig. 5). Contrary to the Al/SiO2 couple, the metallic network in the Al16.7Cu/SiO2 couple is composed of three phases identified as Al2Cu (white), Si (white), and Al(Cu, Si) (gray). Moreover, the large areas corresponding to either the Al2Cu phase or the mixture of Al2Cu and Al(Cu, Si) phases were well distinguished. Their location and distribution in the RPR suggest that in these areas, the Al2Cu and Al(Cu, Si) phases were formed during cooling when the solidification of the metallic network started from the nucleation of the primary Al2Cu phase and finished by the formation of eutectic Al2Cu + Al(Cu, Si).
Another interesting feature of the RPR structure in comparison with the Al/SiO2 system is the presence of the δ-Al2O3 phase observed in the Al16.7Cu/SiO2 couple both in the transition layer, formed between the SiO2 substrate and the RPR, and in few areas inside the RPR, as marked in Fig. 5. Our previous TEM studies of Al/SiO2 couple evidenced only the formation of α-Al2O3 phase of various morphology (large crystals, fibers, and small precipitates). The morphology and crystallography of the alumina in the Al/SiO2 system were extensively studied by Breslin et al. [1], who reported the presence of large α-Al2O3 crystals (several micrometers in size) surrounded by the fine grains of θ-Al2O3 phase in the samples obtained at 1173 K. It was remarked that the growth of α-Al2O3 took place on the expense of the θ-Al2O3. Below this temperature, Breslin et al. described the growth of solely θ-Al2O3, while in a higher temperature range, the α-Al2O3 phase was only identified. It should be emphasized that in both cases, the formation of C4-type structure took place apart from various types of alumina. On the other hand, Yoshikawa et al. [6] identified θ-, γ-, and α-Al2O3 phases growing in the Al/SiO2 at 1073 K, although they observed the microstructure of complex morphology showing a coexistence of both fine grains of θ-Al2O3 phase and large α-Al2O3 crystals (several micrometers in size) after the interaction at 1173 K. Yoshikawa et al. [6] concluded that such a change in the microstructure can be due to the differences in inherent size of the α-Al2O3, as the large grains formed separated regions rather than due to a thermal activation process.
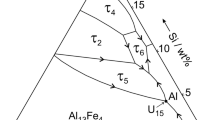
Silicon produced in redox reaction (1) dissolving in the Al–Cu solution changes the Al and Si activity in the liquid solution (denoted by underlined element symbols):
Figure 6 presents the estimated path of the liquid alloy composition change during the redox reaction like it was superimposed in the Al–Cu–Si phase diagram calculated from the assessed binaries using FToxid and SGTE databases [20]. At the beginning of interaction, the liquid alloy should be in equilibrium with the solid Al2O3 polymorphs and solid SiO2. Next, after attaining the liquidus line (Fig. 6), the pure Si begins to precipitate which was also evidenced experimentally based on the results of structural characterization shown in Figs. 2f, 4a, e. Following the equilibrium phase diagram, the mullite phase (Al6Cu2O13) could form when the equilibrium changes to liquid Cu–Si solution, mullite, and pure silicon. However, it might have taken place after the consumption of all aluminum in redox reaction with excesses of SiO2, which was not the case in the present study. Moreover, the Al2Cu (theta-AlCu) phase noted in the solidified sample was formed during its cooling because the Al2Cu phase melts at 880 K which is lower than the test temperature [21, 22]. The precipitation of neither η-AlCu or ε-AlCu phases existing in the Al–Cu–Si phase diagram has been observed in the RPR area, although under the conditions of the present study, the copper content in the solidifying Al–Cu alloy should be lower than 33 at.%.
After 2 h interaction of Al–Cu liquid with the solid SiO2 substrate, the metallic Al was still observed in the RPR; this might be incorrectly interpreted with the remark that the kinetics of exchange reaction is not fast enough. However, the influence of two effects the wettability as well as large volumetric mismatch between substrates and products must be taken into account [5].
In the analysis of the phase transformation in the examined system, the possibility for the formation of metastable phases of aluminum III oxides in the Ostwald’s cascade of stages cannot be excluded. As reported in Ref. [23, 24], the crystallization from a solution occurs in steps in such a way that thermodynamically unstable phases often occur first, followed by the thermodynamically stable ones. Ostwald‘s step rule refers to irreversible thermodynamics, and it has been shown that it minimizes entropy production. According to Ostwald step rule, the less stable structures of Al2O3 can be formed step by step in direction to stable polymorph, which simulates the reversible conditions, and thus minimizes the entropy production. In the present study on the thermodynamic reaction between the Al–Cu liquid alloy and solid SiO2, it was essential to check which phase would be formed before the stable alpha appearance. The alpha was suspended into thermodynamic calculations (FactSage 6.3, GTT-Technologies, Germany [25]) which showed that the solid δ-Al2O3 could appear first.
The Ostwald rule is valid not only for the systems far from equilibrium, but also for systems under steady-state conditions, which were obtained in the described experiments during continuous pumping. A detailed equilibrium thermodynamic description is not possible, but it is feasible to estimate some reaction products. With the direct contact of Al–Cu with SiO2, the redox reaction occurred, and Si was deposited together with the metastable δ-Al2O3.
Furthermore, in the transition layer, formed in the vicinity of SiO2 substrate, as well as in the RPR, the fine δ-Al2O3 precipitates are surrounded with very fine intermixed precipitates of Al2Cu and Si phases only (Fig. 5). Thus, we suggest that the freshly formed Si does not dissolve, as the transfer of Si from the reaction front is blocked because of a lack of sufficient amount of liquid. Such situation can be explained from point of the view of volumetric changes accompanying the phase transformations in the systems. For the pure Al/SiO2 couple, the transfer of one mole of SiO2 into 2/3 of mole of α-Al2O3 results in 38 % volume decrease. However, compared to stable α-Al2O3 a = b = 4.758 Å; c = 12.991 Å [26] the metastable δ-Al2O3 phase (a = 7.9 Å; b = 15.8 Å; c = 11.85 Å) has 5.02 times higher volume. Therefore, at least at the first stage of interaction, the δ-Al2O3 phase forms continuous and dense layer affecting the kinetics of redox reaction because a lack of open channels in the freshly formed transition layer slows down the transfer of the Al and Si to and from the reaction front, respectively. After reaching a critical thickness, the further growth of this layer starts to crack because of increase in volume accompanying SiO2 → δ-Al2O3 transformation. It may also cause cracking in the nearest substrate layer resulting in the formation of large fragments of SiO2 surrounded by liquid metal. In the next step, the interaction will take place along the surface of these separated fragments as well as the fresh surface of SiO2 substrate, again starting from the formation of δ-Al2O3. After some exposure time, metastable δ-Al2O3 transforms to stable α-Al2O3. However, this process is accompanied with significant volume decrease, and it results in the formation of the network of discontinuities in the α-Al2O3 layer and liquid metal penetration into the channels formed. This explanation is in a good agreement with structural observations on the appearance of δ-Al2O3 either in the transition layer between the SiO2 substrate and the RPR or in the areas inside the RPR where the fragmentation of the unreacted substrate took place.
The presence of few large cracks was noted inside the substrate but in the vicinity of the RPR similar to the Al/SiO2 couple. Based on the direct visual observation of the interaction between the transparent SiO2 plates and liquid Al drop reported by Sobczak et al. [27], it is concluded that such cracks are formed during cooling of the couple and they are caused by the stresses produced because of significant CTE mismatch of the materials involved. On the contrary, as evidenced by Sobczak et al. [27], the formation of cracks inside the RPR in the Al/SiO2, taking place directly during high-temperature interaction, is attributed to the volumetric mismatch between initial reactants and the reaction products in the Al/SiO2 couple.
A common feature was noted in the Al/SiO2 and Al16.7Cu/SiO2 couples, i.e., the presence of individual large α-Al2O3 crystals at the drop-side RPR interface (see Fig. 1b). Similar features were also reported in both the reactive Al/oxide systems (e.g., Al/mullite, Al/kaolin, Al/NiO, Al/CoO, and Al/TiO2 [2, 3, 9, 28]) and non-reactive ones (e.g., Al/Al2O3, AlSi/Al2O3, and AlCu/Al2O3) [2, 3]. As suggested by Sobczak [2], different mechanisms are responsible for the dissimilar morphology of alumina formed, i.e., the smaller precipitates in the RPR inside the substrate are formed through direct redox reaction while the bigger crystals at the drop-side interface are formed through dissolution–precipitation mechanism. The nucleation of large alumina crystals at the drop-side RPR interface, according to Avraham and Kaplan [29], is explained by the transfer of oxygen from surrounding atmosphere due to the local high oxygen partial pressure. However, there are at least two reasons that confirm the dissolution–precipitation mechanism of their formation: (1) both the size and the amount of these crystals increase from the periphery to the center of the drop, and (2) the formation of similar crystals of AlN phase (but not the alumina one), which was also observed in Al/AlN sessile drop couples produced under similar testing conditions.
Summary
This study characterizes the reaction products formed between the liquid Al–Cu alloy (16.7 at.% of Cu) and SiO2 at 1173 K under vacuum after 2 h of interaction. The creation of three different subzones within the silica substrate took place. A layer of about 30 μm in thickness composed of large Al2O3 crystals surrounded by the Al2Cu phase was extended below the drop. These two phases formed mutually interpenetrating network below the mentioned layer; however, their morphologies were different. Selected area diffraction patterns confirmed the assumptions that the formed alumina was of the corundum structure. Moreover, silicon was present within this subzone either in the dissolved state in Al2O3 and Al2Cu or as a separate precipitate.
On the other hand, areas of very fine-grained microstructure could be also found, next to the silica and within the RPR. The matrix consisted of the orthorhombic δ-Al2O3 and was surrounded by the grains of Al2Cu and Si of 100–200 nm in size. The volumetric mismatch between SiO2 and Al2O3 caused not only the cracks inside the substrate and close to its interface with RPR, but also the appearance of deformation twins in silicon, twinned on (1–11) plane.
References
Breslin MC, Ringnala J, Seeger J, Marasco AL, Daehn GS, Fraser HL (1994) Cer Eng Sci Proc 15(4):104
Sobczak N (2005) Solid State Phenom 101–102:221
Sobczak N (2006) Interaction between molten aluminum and oxides. In: Gupta N, Hunt WH (eds) Solidification processing of metal matrix composites. TMS publications, Ohio, pp 133–146
Yoshikawa N, Kikuchi A, Taniguchi S (2000) Mater Trans 41(3):399
Sobczak N, Ksiazek M, Radziwill W, Warmuzek M, Nowak R, Kudyba A (2003) Effect of temperature and alloying additions on wettability and reactivity in Al/SiO2 system. Odlewnictwo—Nauka i Praktyka 3:3–14
Sandhage KH (2010) JOM 62(6):32
Strange AC, Breslin MC (1998) U.S. patent 5,728,638
Evarts JS (2008) M.S. Thesis, The Ohio State University, Ohio
Sobczak N, Stobierski L, Ksiazek M, Radziwill W, Nowak R, Kudyba A (2003) Ceramika—polish ceramic bullletin 80:831
Sobczak N, Sobczak J, Morgiel J, Stobierski L (2003) Mater Chem Phys 81:296
Shen P, Fujii H, Matsumoto T, Nogi K (2004) Acta Mater 52:887
Nowak R, Sobczak N, Sienicki E, Morgiel J (2011) Solid State Phenom 172–174:1273
Wojewoda-Budka J, Sobczak N, Morgiel J, Nowak R (2010) J Mater Sci 45(16):4291. doi:10.1007/s10853-010-4379-6
Wojewoda-Budka J, Sobczak N, Morgiel J, Nowak R (2011) Solid State Phenom 172–174:1267
Liang WW (1982) Z Metallkd 73(6):369
Yi S, Trumble KP, Gaskell DR (1999) Acta Mater 47(11):3221
Meetsma A, De Boer JL, Van Smaalen S (1989) J Solid State Chem 83:370
Lebeau T, Srom-Olsen JO, Gruzelski JE, Drew RA (1995) Mater Charact 35:11
Levin I, Brandon D (1998) J. Am. Ceram. Soc. 81(8):1995
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Hack K, Jung I-H, Kang Y-B, Melançon J, Pelton AD, Robelin C, Petersen S (2009) Calphad 33(2):295
Liang H, Chang YA (1998) J Phase Equilib 19:25
Witusiewicz VT, Hecht U, Fries SG, Rex S (2004) J. Alloys Comp 385:133
van Santen RA (1984) J Phys Chem 88:5768
van Santen RA (1988) J Phys Chem 92:248
Bale CW, Chartrand P, Degterov SA, Eriksson G, Hack K, Ben Mahfoud R, Melançon J, Pelton AD, Petersen S (2002) Calphad 26(2):189
Dogan I (2008) Fabrication and characterization of aluminum oxide and silicon/aluminum oxide films with Si nanocrystals formed by magnetron co-sputtering technique. PhD Thesis, Physics Department, Middle East Technical University, Ankara
Sobczak N, Asthana R, Radziwill W, Nowak R, Kudyba A (2007) Arch Metall Mater 52(1):55
Sobczak N, Oblakowski J, Nowak R, Kudyba A, Radziwill W (2005) J Mater Sci 40(9/10):2313. doi:10.1007/s10853-005-1951-6
Avraham S, Kaplan WD (2005) J Mater Sci 40(5):1093. doi:10.1007/s10853-005-6922-4
Acknowledgements
This study has been supported by the Ministry of Science and Higher Education of Poland within the Project No. N N507 272836.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wojewoda-Budka, J., Sobczak, N., Litynska-Dobrzynska, L. et al. TEM characterization of the reaction products formed in Al–Cu/SiO2 couples due to high temperature interaction. J Mater Sci 47, 8464–8471 (2012). https://doi.org/10.1007/s10853-012-6798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6798-z