Abstract
Three types of host molecules 1–3 based on a phenolphthalein (one of the most popular pH indicators) and two crown ethers were prepared for use in colorimetric recognition of linear diamines, triamines, sequence of non-protected dipeptides in methanol or water solution.




















Similar content being viewed by others
Notes
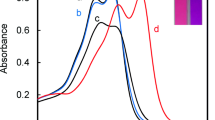
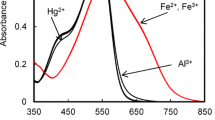
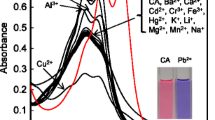
In Fig. 9, the proposed colored complex between host 1 and guest 13 is shown as canonical structure. Therefore, there may be some irrational interaction between the quinoid form of the phenolic crown ether of the host and the neutral amino group of the guest. We can interpret the actual complex as follows: (i) the guest amine reacts with free host 1 to generate two kinds of dianions, (ii) with regard to “colored” complex, one anion preferentially locates on the carboxylate, and another anion spreads over the two phenolic rings, and (iii) one proton is exchanged between the corresponding two amines through surrounding methanol, and thus both amines acquire a cationic character (No coloration was observed in a protic polar solvents, such as CHCl3, DMSO, and DMF, see Fig. 16).
References
de Silva, A.P., Gunaratne, H.Q.N., Gunnlaugsson, T,. Huxley, A.J.M., McCoy, C.P., Rademacher, J.T., Rice, T.E.: Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997)
McQuade, D.T., Pullen, A.E., Swager, T.M.: Conjugated polymer-based chemical sensors. Chem. Rev. 100, 2537–2574 (2000)
Wiskur, S.L., Ait-Haddou, H., Lavigne, J.L., Anslyn, E.V.: Teaching old indicators new tricks. Acc. Chem. Res. 34, 963–972 (2001)
Kuwabara, T.: Color change indicator for molecules. J. Jpn. Soc. Colour Mater. 78, 265–271 (2005)
Nguyen, B.T., Anslyn, E.V.: Indicator-displacement assays. Coord. Chem. Rev. 250, 3118–3127 (2006)
Bubnis, B.P., Pacey, G.E.: Alkali metal ion complexation with lariat ethers possessing a chromogenic group. Talanta 31, 1149–1152 (1984)
Nakamura, H., Takagi, M., Ueno, K.: Complexation and extraction of alkali metal ions by 4′-picrylaminobenzo-18-crown-6 derivatives. Anal. Chem. 52, 1668–1671 (1980)
Bubnis, B.P., Steger, J.L., Wu, Y.P., Meyers, L.A., Pacey, G.E.: Substituent effects on complexation and extraction of alkali metals with chromogenic crown ethers. Anal. Chim. Acta. 139, 307–313 (1982)
Fuji, K., Tsubaki, K., Tanaka, K., Hayashi, N., Otsubo, T., Kinoshita, T.: Visualization of molecular length of α,ω-diamines and temperature by a receptor based on phenolphthalein and crown ether. J. Am. Chem. Soc. 121, 3807–3808 (1999)
Tsubaki, K., Hayashi, N., Nuruzzaman, M., Kusumoto, T., Fuji, K.: Visual recognition of triamines by phenolphthalein derivatives: consideration of the structure of the colored complex. Org. Lett. 3, 4067–4069 (2001)
Tsubaki, K., Nuruzzaman, M., Kusumoto, T., Hayashi, N., Wang, B.-G., Fuji, K.: Visual enantiomeric recognition using chiral phenolphthalein derivatives. Org. Lett. 3, 4071–4073 (2001)
Tsubaki, K., Kusumoto, T., Hayashi, N., Nuruzzaman, M., Fuji, K.: Sequence-selective visual recognition of nonprotected dipeptides. Org. Lett. 4, 2313–2316 (2002)
Tsubaki, K., Tanima, D., Nuruzzaman, M., Kusumoto, T., Fuji, K., Kawabata, T.: Visual enantiomeric recognition of amino acid derivatives in protic solvents. J. Org. Chem. 70, 4609–4616 (2005)
Tsubaki, K., Tanima, D., Kuroda, Y., Fuji, K., Kawabata, T.: Bidirectional and colorimetric recognition of sodium and potassium ions. Org. Lett. 8, 5797–5800 (2006)
Tsubaki, K., Tanima, D., Sasamori, T., Tokitoh, N., Kawabata, T.: Colorimetric recognition of the length of α,ω-diamines in water. Tetrahedron Lett. 48, 2135–2138 (2007)
Tsubaki, K., Tanima, D., Imamura, Y., Kawabata, T. (submitted)
Tamura, Z., Abe, S., Ito, K., Maeda, M.: Spectrophotometric analysis of the relationship between dissociation and coloration, and of the structural formulas of phenolphthalein in aqueous solution. Anal. Sci. 12, 927–930 (1996)
Taguchi, K.: Transient binding of phenolphthalein-β-cyclodextrin complex: an example of induced geometrical distortion. J. Am. Chem. Soc. 108, 2705–2709 (1986)
Kuwabara, T., Takamura, M., Mastushita, A., Ikeda, H., Nakamura, A., Ueno, A., Toda, F.: Phenolphthalein-modified β-cyclodextrin as a molecule-responsive colorless-to-color change indicator. J. Org. Chem. 63, 8729–8735 (1998)
For a recent review for temperature effect on the selectivity: Buschmann, H., Scharf, H.-D., Hoffmann, N., Esser, P.: The isoinversion principle. A general selection model in chemistry. Angew. Chem. Int. Ed. Engl. 30, 477–515 (1991)
Naemura, K., Tobe, Y., Kaneda, T.: Preparation of chiral and meso-crown ethers incorporating cyclohexane-1,2-diol derivatives as a steric barrier and their complexation with chiral and achiral amines. Coord. Chem. Rev. 148, 199–219 (1996)
Kaneda, T., Sugihara, K., Kamiya, H., Misumi, S.: Synthetic macrocyclic ligands. IV. Lithium ion-characteristic coloration of a “crowned” dinitrophenylazophenol. Tetrahedron Lett. 22, 4407–4408 (1981)
Kaneda, T., Umeda, S., Ishizaki, Y., Kuo, H.S., Misumi, S., Kai, Y., Kanehisa, N., Kasai, N.: Azophenolic acerands: amine-selective coloration and crystal structure of a piperidinium saltex. J. Am. Chem. Soc. 111, 1881–1883 (1989)
Kaneda, T., Hirose, K., Misumi, S.: Chiral azophenolic acerands: color indicators to judge the absolute configuration of chiral amines. J. Am. Chem. Soc. 111, 742–743 (1989)
Naemura, K., Ueno, K., Takeuchi, S., Tobe, Y., Kaneda, T., Sakata, Y.: Azophenolic acerands having chiral 1-phenyl-cis-1,2-cyclohexanediol units: a correlation between enantiorecognitive coloration and host–guest complementarity. J. Am. Chem. Soc. 115, 8475–8476 (1993)
Hirose, K., Fuji, J., Kamada, K., Tobe, Y., Naemura, K.: Temperature dependent inversion of enantiomer selectivity in the complexation of optically active azophenolic crown ethers containing alkyl substituents as chiral barriers with chiral amines. J. Chem. Soc. Perkin Trans. 2, 1649–1657 (1997)
Ogasahara, K., Hirose, K., Tobe, Y., Naemura, K.: Preparation of optically active azophenolic crown ethers containing 1-phenylethane-1,2-diol and 2,4-dimethyl-3-oxapentane-1,5-diol as a chiral subunit: temperature-dependent enantiomer selectivity in the complexation with chiral amines. J. Chem. Soc. Perkin Trans. 1, 3227–3236 (1997)
Hirose, K., Ogasahara, K., Nishioka, K., Tobe, Y., Naemura, K.: Enantioselective complexation of phenolic crown ethers with chiral aminoethanol derivatives: effects of substituents of aromatic rings of hosts and guests on complexation. J. Chem. Soc. Perkin Trans. 2, 1984–1993 (2000)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Rose, N.J., Drago, R.S.: Molecular addition compounds of iodine. I. An absolute method for the spectroscopic determination of equilibrium constants. J. Am. Chem. Soc. 81, 6138–6145 (1959)
Hirose, K.J.: Inclusion phenom.: a practical guide for the determination of binding constants. Macrocyclic Chem. 39, 193–209 (2001)
Still, W.C.: Discovery of sequence-selective peptide binding by synthetic receptors using encoded combinatorial libraries. Acc. Chem. Res. 29, 155–163 (1996)
Hossain, M.A., Schneider, H.-J.: Sequence-selective evaluation of peptide side-chain interaction. New artificial receptors for selective recognition in water. J. Am. Chem. Soc. 120, 11208–11209 (1998)
Tashiro, S., Tominaga, M., Kawano, M., Therrien, B., Ozeki, T., Fujita, M.: Sequence-selective recognition of peptides within the single binding pocket of a self-assembled coordination cage. J. Am. Chem. Soc. 127, 4546–4547 (2005)
Wehner, M., Janssen, D., Schaefer, G., Schrader, T.: Sequence-selective peptide recognition with designed modules. Eur. J. Org. Chem. 138–153 (2005)
Bush, M.E., Bouley, N.D., Urbach, A.R.: Charge-mediated recognition of N-terminal tryptophan in aqueous solution by a synthetic host. J. Am. Chem. Soc. 127, 14511–14517 (2005)
Israelachvili, J.N.: Intermolecular and Surface Forces. Academic Press, London (1992)
Acknowledgments
The author thanks all collaborators mentioned in the references. He especially thanks Prof. Kaoru Fuji and Prof. Takeo Kawabata for their suggestions and discussions, Dr. Daisuke Tanima, Dr. Tomokazu Kusumoto, Dr. Mohammad Nuruzzaman, Mr. Noriyuki Hayashi, and Ms. Yoko Imamura for their great contributions to this work. This study was partly supported by Grants-in-Aid for Scientific Research (Nos. 14572003 and 18390003) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Foundation for Pharmaceutical Sciences, Japan. He also thanks Prof. Norihiro Tokitoh and Prof. Takahiro Sasamori (Kyoto Univ.) for performing the X-ray structural analysis, and Prof. Yasuhisa Kuroda (Kyoto Institute of Technology), Prof. Keiji Hirose and Prof. Yoshito Tobe (Osaka Univ.) for gifts of software for determination of binding constants. This is a paper selected for “HGCS Japan Award of Excellence 2007”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsubaki, K. Colorimetric recognition using functional phenolphthalein derivatives. J Incl Phenom Macrocycl Chem 61, 217–225 (2008). https://doi.org/10.1007/s10847-008-9419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9419-3